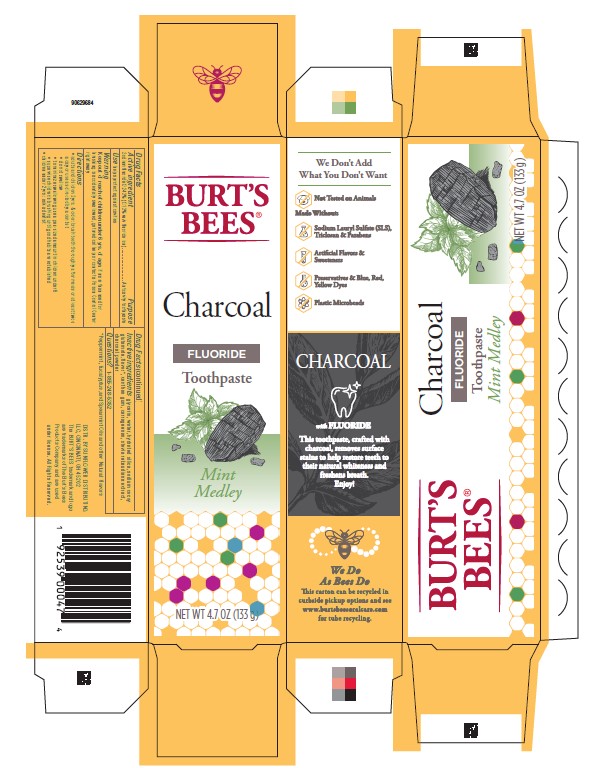
BURTS BEES CHARCOAL MINT MEDLEY- sodium fluoride paste, dentifrice
Procter & Gamble Manufacturing Company
----------
Burt's Bees® Charcoal Mint Medley
Directions
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6
- supervise children’s brushing until good habits are established
- children under 2 yrs.: ask a dentist
| BURTS BEES
CHARCOAL MINT MEDLEY
sodium fluoride paste, dentifrice |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Company (004238200) |
Revised: 10/2023
Document Id: 06fcc643-ca44-6857-e063-6394a90a67e1
Set id: 9f05f4f1-18fc-4d55-e053-2a95a90a4f85
Version: 4
Effective Time: 20231005
Procter & Gamble Manufacturing Company