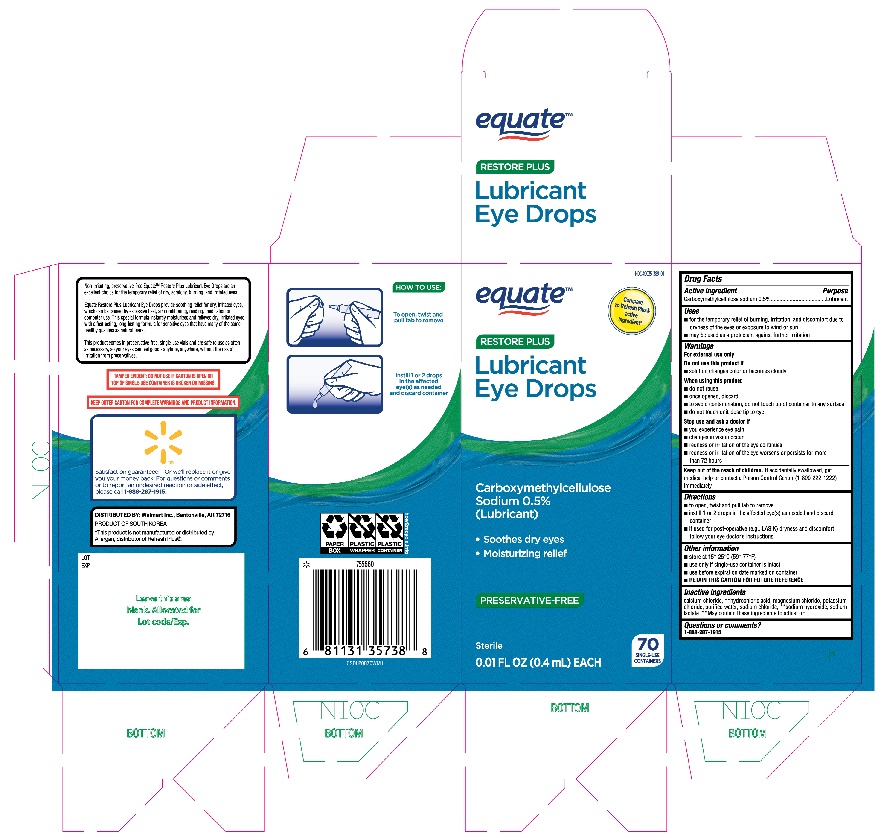
Label: EQUATE RESTORE PLUS LUBRICANT EYE DROPS- carboxymethylcellulose sodium solution/ drops
- NDC Code(s): 49035-389-01
- Packager: Walmart, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 23, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
When using this product
- do not reuse
- once opened, discard
- to avoid contamination, do not touch tip of container to any surface
- do not touch unit-dose tip to eye
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- Equate Restore Plus Lubricant Eye Drops 70ct
-
INGREDIENTS AND APPEARANCE
EQUATE RESTORE PLUS LUBRICANT EYE DROPS
carboxymethylcellulose sodium solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-389 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) (CARBOXYMETHYLCELLULOSE - UNII:05JZI7B19X) CARBOXYMETHYLCELLULOSE SODIUM 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM LACTATE (UNII: TU7HW0W0QT) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-389-01 70 in 1 CARTON 03/04/2022 1 0.4 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 03/04/2020 Labeler - Walmart, Inc. (051957769) Registrant - K.C. Pharmaceuticals, Inc. (174450460) Establishment Name Address ID/FEI Business Operations K.C. Pharmaceuticals, Inc. 174450460 pack(49035-389) , label(49035-389) Establishment Name Address ID/FEI Business Operations Unimed Pharmaceuticals, Inc. 689852052 manufacture(49035-389)