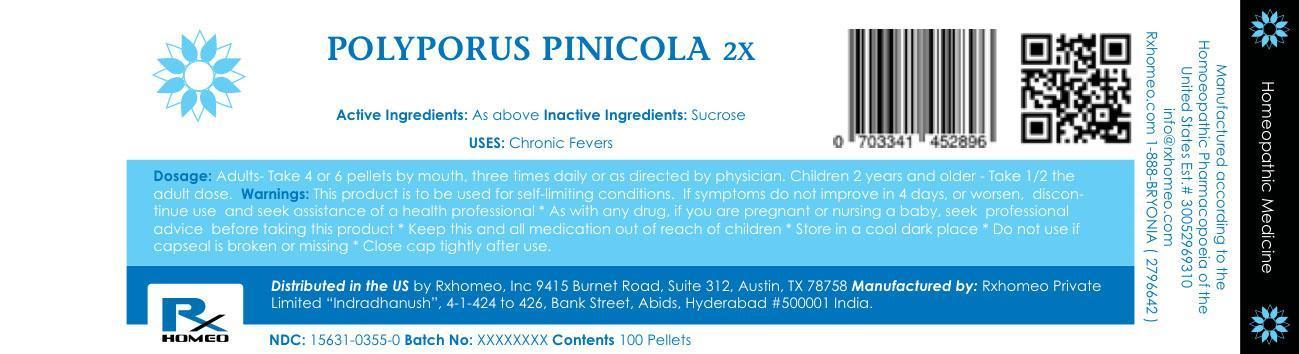
Label: POLYPORUS PINICOLA pellet
-
Contains inactivated NDC Code(s)
NDC Code(s): 15631-0355-0, 15631-0355-1, 15631-0355-2, 15631-0355-3, view more15631-0355-4, 15631-0355-5 - Packager: Rxhomeo Private Limited d.b.a. Rxhomeo, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 1, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- USES
- INDICATIONS
- DOSAGE
- WARNINGS
- INACTIVE INGREDIENTS
-
QUESTIONS OR COMMENTS
www.Rxhomeo.com | 1.888.2796642 | info@rxhomeo.com
Rxhomeo, Inc 9415 Burnet Road, Suite 312, Austin, TX 78758
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
POLYPORUS PINICOLA
polyporus pinicola pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:15631-0355 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOMITOPSIS PINICOLA FRUITING BODY (UNII: 30D02U2IRN) (FOMITOPSIS PINICOLA FRUITING BODY - UNII:30D02U2IRN) FOMITOPSIS PINICOLA FRUITING BODY 2 [hp_X] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15631-0355-0 100 in 1 PACKAGE; Type 0: Not a Combination Product 2 NDC:15631-0355-1 200 in 1 PACKAGE; Type 0: Not a Combination Product 3 NDC:15631-0355-2 400 in 1 PACKAGE; Type 0: Not a Combination Product 4 NDC:15631-0355-3 750 in 1 PACKAGE; Type 0: Not a Combination Product 5 NDC:15631-0355-4 2500 in 1 PACKAGE; Type 0: Not a Combination Product 6 NDC:15631-0355-5 12500 in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/12/2015 Labeler - Rxhomeo Private Limited d.b.a. Rxhomeo, Inc (650833994) Establishment Name Address ID/FEI Business Operations Rxhomeo, Inc 832534981 wholesale drug distributor(15631-0355) Establishment Name Address ID/FEI Business Operations Rxhomeo Private Limited d.b.a. Rxhomeo, Inc 650833994 manufacture(15631-0355) , label(15631-0355)