DEODERM GEL DRESSING WITH HYALURONIC ACID- dressing, wound and burn, occlusive
LIFSA DRUG LLC
----------
Deoderm Gel Dressing with Hyaluronic Acid
DESCRIPTION
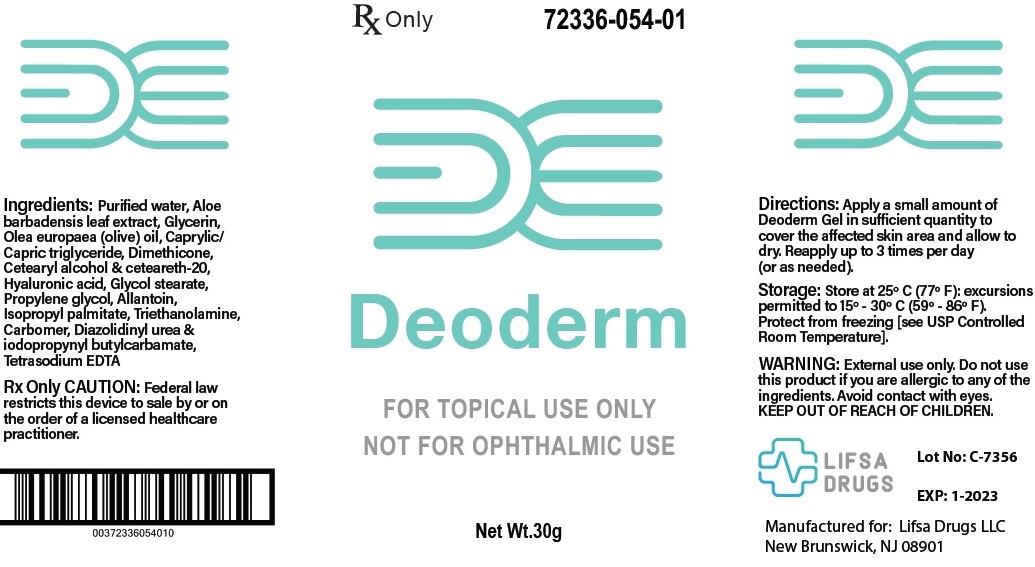
Deoderm Gel is a soothing wound gel that is formulated for the dressing and management of superficial wounds, minor abrasions, pressure ulcers stage II - IV, 1st and 2nd degree burns, including sunburns, and radiation dermatitis. When applied properly to a wound hyaluronic acid adheres to tissue to prevent damage while supplying moisture to the cells. Medium molecular weight polysaccharides in aloe vera moisturize and reduce inflammation. Proven therapeutic moisturizers, lipids, glycerin and allantoin, relieves dryness.
Deoderm Gel is a non-sterile, off-white, low odor, fragrance free, topical product. The Deoderm Gel forms a physical barrier that helps to maintain a moist wound and skin environment. Deoderm Gel is a prescription medical device.
INDICATIONS FOR USE
Under the supervision of a healthcare professional, Deoderm Gel is indicated to manage and relieve the burning, itching and pain experienced with various types of dermatoses, including atopic dermatitis, allergic contact dermatitis and radiation dermatitis. Deoderm Gel also helps to relieve dry, waxy skin by maintaining a moist wound and skin environment, which is beneficial to the healing process.
CONTRAINDICATIONS
Deoderm Gel is contraindicated in persons with a known hypersensitivity to any of the components of the formulation.
WARNINGS
Use only as directed. Keep out of the reach of children. Avoid contact with eyes. For topical use only. Not for ophthalmic use. Do not apply within four hours prior to a radiation session.
PRECAUTIONS AND OBSERVATIONS
- Deoderm Gel does not contain a sunscreen and should not be used prior to extended exposure to the sun.
- If clinical signs of infection are present, appropriate treatment should be initiated; use of Deoderm Gel may be continued during the anti-infective therapy.
INSTRUCTIONS FOR USE
Dispense Deoderm Gel into palm of hand and apply to affected area 3 times per day, or as directed by a physician. Massage gently into the skin until completely absorbed. If the skin is broken, cover with appropriate dressing Deoderm Gel is non-sterile.
INGREDIENTS
Allantoin
Aloe Barbadensis Leaf Extract
Caprylic/Capric Triglyceride
Carbomer
Ceteareth 20
Cetearyl Alcohol
Diazolidinyl Urea & Iodopropynyl Butylcarbamate
Dimethicone
Glycerin
Glycol Stearate
Hyaluronic Acid
Isopropyl Palmitate
Olea Europaea (Olive) Oil
Propylene Glycol
Tetrasodium EDTA
Triethanolamine
Water
| DEODERM
GEL DRESSING WITH HYALURONIC ACID
dressing, wound and burn, occlusive |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - LIFSA DRUG LLC (081205160) |