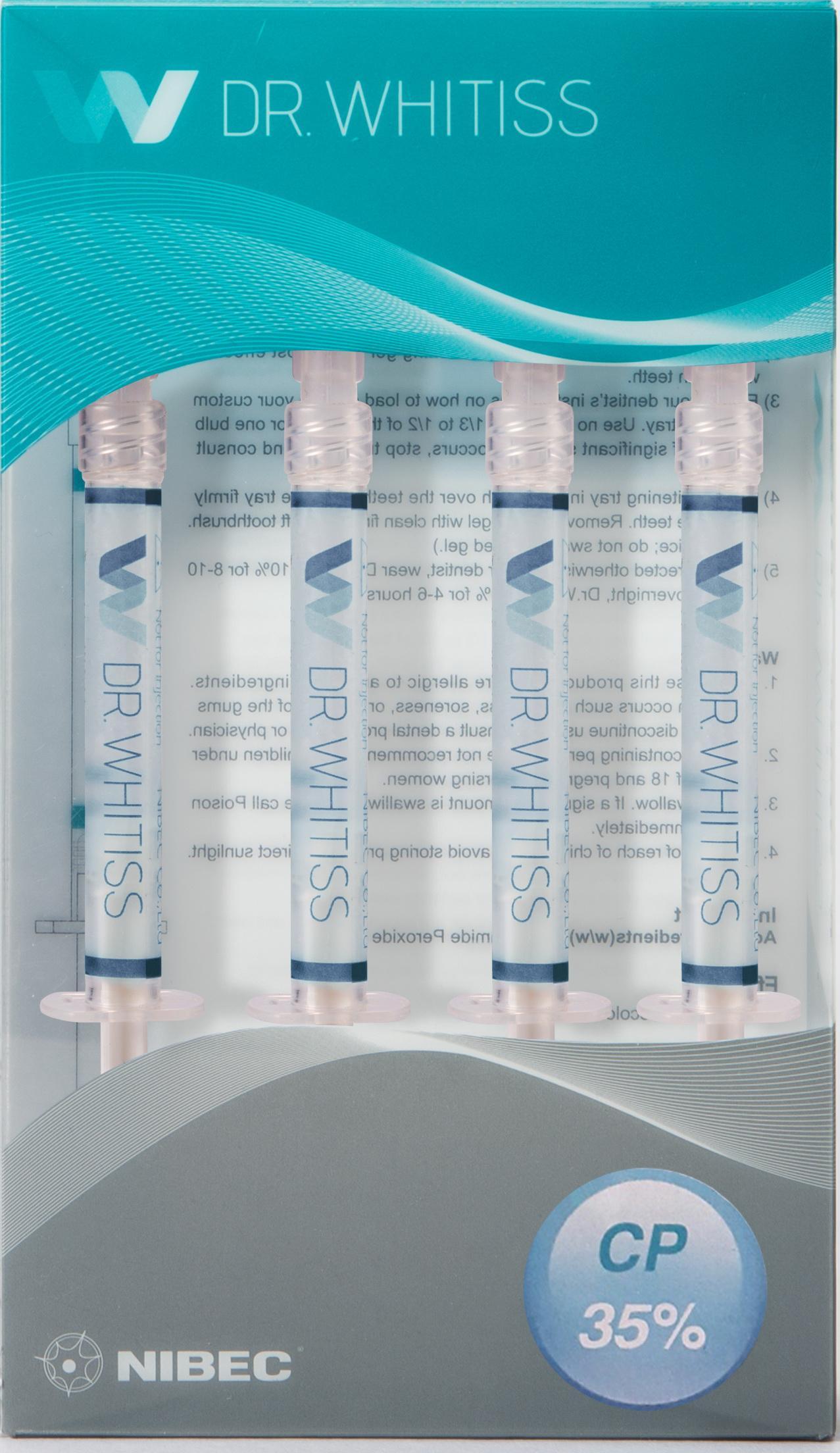
Label: DR.WHITISS 35%- carbamide peroxide gel, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 47649-1301-1, 47649-1301-2 - Packager: Nibec Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 26, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
1. Gingival and general oral heath should be confirmed before treatment.
2. Brush your teeth before treatment.
3. Follow your dentist’s instructions on how to load gel into your custom whitening tray. Use no more than 1/3 to 1/2 of the syringe per tray.
4. Insert whitening tray in the mouth over the teeth. Seat the tray firmly agains the teeth. Remove excess gel with clean finger or soft toothbrush.(Rinse twice; do not swallow rinsed gel.)
5. Unless directed otherwise by your dentist, wear Dr.Whitiss 10% for 8-10 hours or overnight, Dr.Whitiss 15% for 4-6 hours, Dr.Whitiss20% for 2-4 hours, and Dr.Whitiss 35% for 30 minutes.
6. If significant sensitivity occurs, stop treatment and consult dentist.
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR.WHITISS 35%
carbamide peroxide gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47649-1301 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 35 g in 100 g Inactive Ingredients Ingredient Name Strength POVIDONE K90 (UNII: RDH86HJV5Z) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) POTASSIUM NITRATE (UNII: RU45X2JN0Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47649-1301-2 4 in 1 PACKAGE 08/11/2015 1 NDC:47649-1301-1 1 g in 1 SYRINGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 08/11/2015 Labeler - Nibec Co., Ltd (687796909) Registrant - Nibec Co., Ltd (687796909) Establishment Name Address ID/FEI Business Operations Nibec Co., Ltd 687796909 manufacture(47649-1301)