GLYTONE SUNVANISH SKIN LIGHTENING- hydroquinone cream
Pierre Fabre USA Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Glytone Clarifying SunVanish with Sunscreen
Glytone SunVanish
HYDROQUINONE USP, 4%
Skin Lightening Cream
with Broad Spectrum SPF 25
Rx Only
FOR EXTERNAL USE ONLY
NOT FOR OPHTHALMIC USE
I. DESCRIPTION
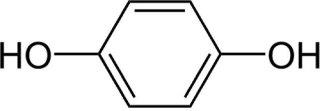
Each gram of Glytone SunVanish (Hydroquinone USP, 4%) Skin Lightening Cream with Broad Spectrum SPF 25 contains 40 mg of Hydroquinone USP with 20 mg of Avobenzone, 75 mg of Octinoxate, 18.6 mg of Octocrylene, and 50 mg Oxybenzone in a cream base of Cetearyl Alcohol, Glycerin, Edetate Disodium, Isopropyl Palmitate, Propylene Glycol, Purified Water, Sodium Lauryl Sulfate, Sodium Metabisulfite, and Sorbic Acid. The chemical name for hydroquinone is: 1,4-benzenediol. The molecular formula is C 6H 6O 2 and molecular weight is 110.11. Hydroquinone has the following structural formula:
II. CLINICAL PHARMACOLOGY
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3, 4-dihydroxyphenylalanine (dopa) and suppression of other melanocyte metabolic processes. Exposure to sunlight or ultraviolet light will cause repigmentation which may be prevented by the broad spectrum sunscreen agents contained in SunVanish.
III. INDICATIONS AND USAGE
Glytone SunVanish is indicated for the gradual lightening of hyperpigmented skin conditions such as chloasma, melasma, freckles, senile lentigines, and other unwanted areas of melanin hyperpigmentation.
IV. CONTRAINDICATIONS
Prior history of sensitivity or allergic reaction to this product or any of its ingredients. The safety of topical hydroquinone use during pregnancy or in children (12 years and under) has not been established.
V. WARNINGS
A. Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions (e.g. hives, itching, wheezing, anaphylaxis, severe asthma attack) in certain susceptible persons. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
B. May produce exogenous ochronosis, a gradual blue-black darkening of the skin, the occurrence of which should prompt discontinuation of treatment.
C. Sunscreen use is an essential aspect of hydroquinone therapy because minimal sunlight sustains melanocytic activity. The sunscreen agents in Glytone SunVanish provide sun protection during skin lightening therapy. During the day, supplement therapy by using an effective broad spectrum sunscreen and avoiding unnecessary sun exposure, or wear sun-protective clothing to cover treated areas in order to prevent repigmentation from occurring.
VI. PRECAUTIONS
See WARNINGS
A. Test for skin sensitivity before using Glytone SunVanish by applying a small amount to an unbroken patch of skin and check in 24 hours. Minor redness is not a contraindication, but where there is itching or vesicle formation or excessive inflammatory response, further treatment is not advised. Close patient supervision is recommended.
Hydroquinone is a skin lightening agent which may produce unwanted cosmetic effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this medication.
B. Avoid contact with eyes, nose, mouth, and lips. In case of accidental contact, patient should rinse thoroughly with warm water and contact a physician.
C. Keep this and all medication out of the reach of children. In case of accidental ingestion, call a physician or a poison control center immediately.
D. Pregnancy Category C. Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether hydroquinone can cause fetal harm when used topically on a pregnant woman or affect reproductive capacity. It is not known to what degree, if any, topical hydroquinone is absorbed systemically. Topical hydroquinone should not be used on pregnant women.
VII. ADVERSE REACTIONS
No systemic adverse reactions to hydroquinone have been reported. Occasional hypersensitivity (localized contact dermatitis) may occur in which case the medication should be discontinued and the physician notified immediately.
Sensitivity to sodium metabisulfite is a potential. See WARNINGS.
VIII. OVERDOSAGE
There have been no systemic reactions from the use of topical hydroquinone. However, treatment should be limited to relatively small areas of the body at one time since some patients experience a transient skin reddening and a mild burning sensation which does not preclude treatment, but indicates caution is warranted.
IX. DRUG DOSAGE AND ADMINISTRATION
Glytone SunVanish should be applied daily in the morning to affected areas and rubbed in well, or as directed by a physician. If no lightening effect is seen after 2 months of treatment, use of this product should be discontinued.
Limit sunlight exposure during the day by applying an effective broad spectrum sunscreen (SPF 15 or higher) or by wearing sun-protective clothing to cover treated areas.
X. HOW SUPPLIED
SunVanish is available as follows:
SizeNDC Number
Net Wt. 2 Oz. / 56 g tube 64760-702-01
SunVanish should be stored at controlled room temperature 15° - 30° C (59° - 86° F). Do not expose to heat above 86° F or direct sunlight.

Distributed by:
Pierre Fabre USA, Inc.
Parsippany, NJ 07054
Made in U.S.A.
1-800-GLYTONE (459-8663)
Rev. 121118
| GLYTONE SUNVANISH SKIN LIGHTENING
hydroquinone cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Pierre Fabre USA Inc. (117196928) |
