IPRATROPIUM BROMIDE AND ALBUTEROL SULFATE- ipratropium bromide and albuterol sulfate solution
The Ritedose Corporation
----------
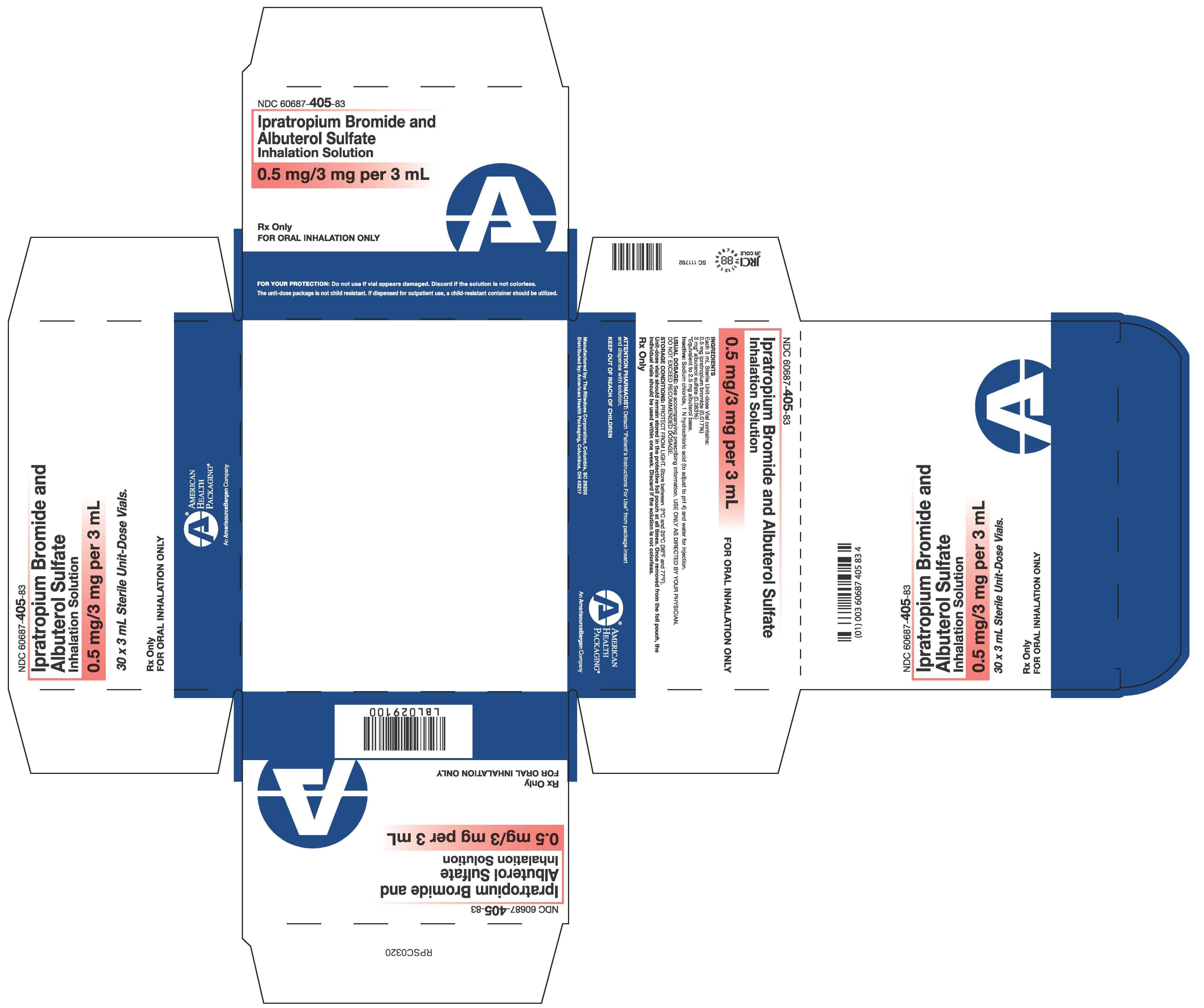
Ipratropium Bromide and Albuterol Sulfate Solution 0.5 mg/3 mg per 3 mL
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution
0.5 mg/3 mg per 3 mL
30 x 3 mL Sterile Unit-Dose Vials.
Rx Only
FOR INHALATION USE ONLY
Principal Display Panel - Ipratropium Bromide and Albuterol Sulfate Inhalation Solution 0.5 mg/3 mg per 3 mL (AHP) 30 ct Brick Carton
NDC 60687- 405-83
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution
0.5 mg/3 mg per 3 mL
30 x 3 mL Sterile Unit-Dose Vials.
Rx Only
FOR INHALATION USE ONLY
Principal Display Panel - Ipratropium Bromide and Albuterol Sulfate Inhalation Solution 0.5 mg/3 mg per 3 mL (GSMS) 30 ct Brick Carton
NDC 60429-975-30
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution
0.5 mg/3 mg per 3 mL
Rx Only
30 x 3 mL Sterile Unit-Dose (1 pouch of 30 - 3 mL vials)

Principal Display Panel - Ipratropium Bromide and Albuterol Sulfate Inhalation Solution 0.5 mg/3 mg per 3 mL (GSMS) 60 ct Brick Carton
NDC 60429-975-60
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution
0.5 mg/3 mg per 3 mL
Rx Only
60 x 3 mL Sterile Unit-Dose Vials (2 pouches of 30 - 3 mL vials)

| IPRATROPIUM BROMIDE AND ALBUTEROL SULFATE
ipratropium bromide and albuterol sulfate solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| IPRATROPIUM BROMIDE AND ALBUTEROL SULFATE
ipratropium bromide and albuterol sulfate solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - The Ritedose Corporation (837769546) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The Ritedose Corporation | 837769546 | analysis(65302-047, 65302-053) , manufacture(65302-047, 65302-053) , pack(65302-047, 65302-053) , label(65302-047, 65302-053) | |