HALOPERIDOL DECANOATE- haloperidol decanoate injection
Zydus Lifesciences Limited
----------
Haloperidol Decanoate Injection
For Intramuscular Injection Only
Rx only
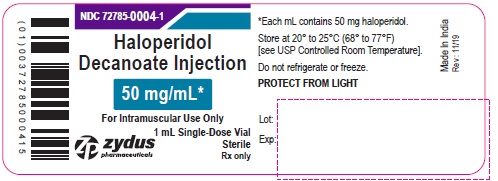
PRINCIPAL DISPLAY PANEL - 50 mg/mL Vial Label
NDC 72785-0004-1
Haloperidol
Decanoate
Injection
50 mg/mL*
For Intramuscular Use Only
1 mL Single-Dose Vial
Sterile
Rx only
zydus pharmaceuticals
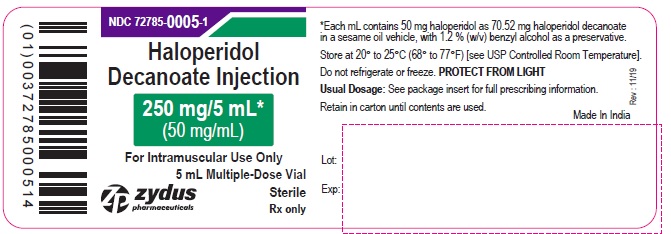
PRINCIPAL DISPLAY PANEL - 250 mg/5 mL (50 mg/mL) Vial Label
NDC 72785-0005-1
Haloperidol
Decanoate Injection
250 mg/5 mL*
(50 mg/mL)
For Intramuscular Use Only
5 mL Multiple-Dose Vial
Sterile
Rx only
zydus pharmaceuticals
PRINCIPAL DISPLAY PANEL - 100 mg/mL Vial Label
NDC 72785-0006-1
Haloperidol
Decanoate
Injection
100 mg/mL*
For Intramuscular Use Only
1 mL Single-Dose Vial
Sterile
Rx only
zydus pharmaceuticals

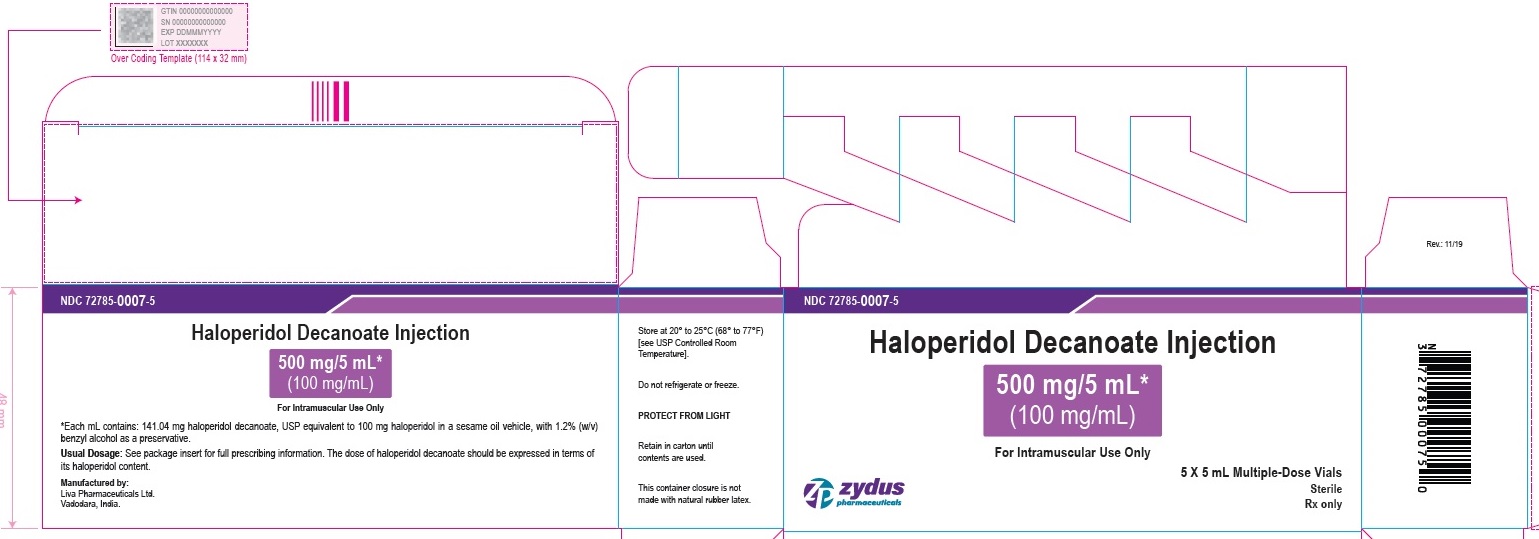
PRINCIPAL DISPLAY PANEL - 500 mg/5 mL (100 mg/mL) Vial Label
NDC 72785-0007-1
Haloperidol
Decanoate Injection
500 mg/5 mL*
(100 mg/mL)
For Intramuscular Use Only
5 mL Multiple-Dose Vial
Sterile
Rx only
zydus pharmaceuticals

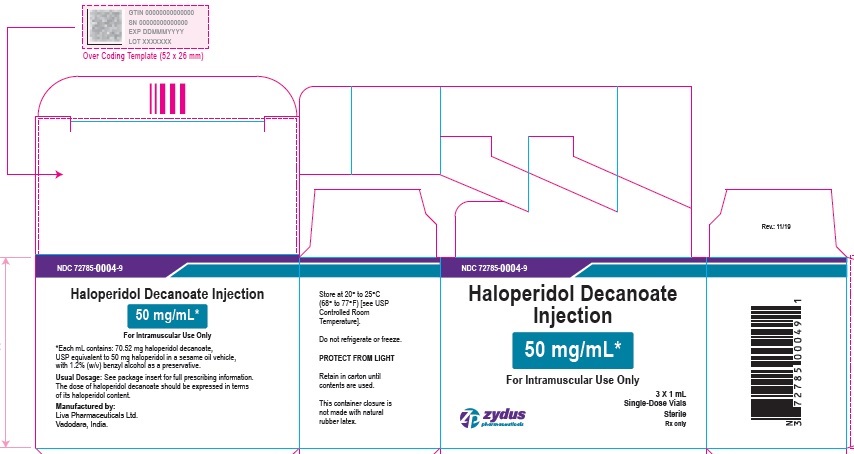
50 mg/mL Carton (3 vials per carton)
NDC 72785-0004-9
Haloperidol Decanoate
Injection
50 mg/mL*
For Intramuscular Use Only
3 X 1 mL
Single-Dose Vials
Sterile
Rx only
zydus pharmaceuticals
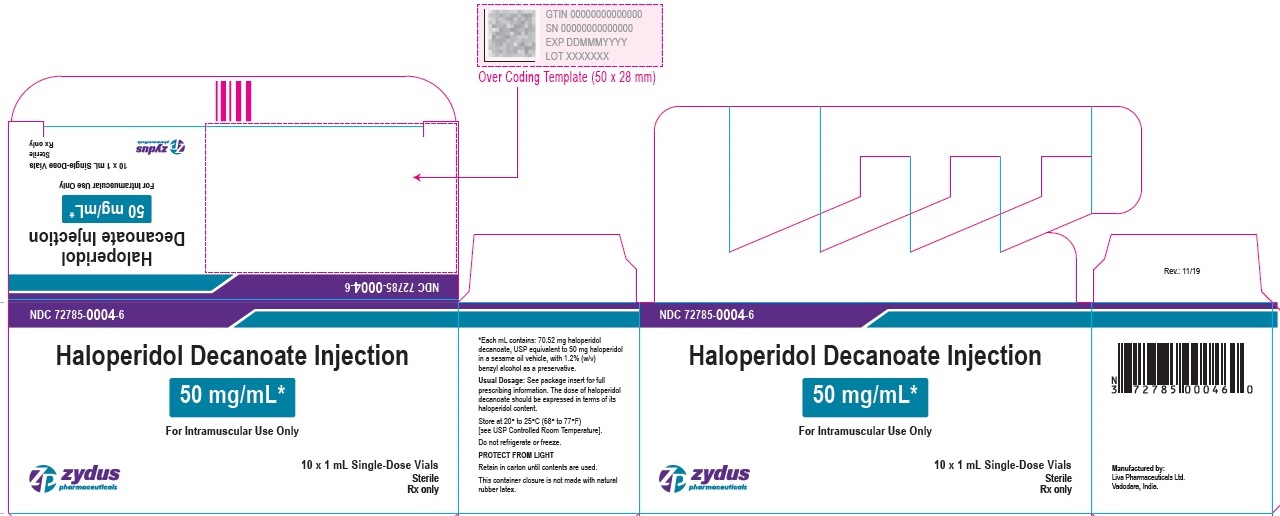
50 mg/mL Carton (10 vials per carton)
NDC 72785-0004-6
Haloperidol Decanoate
Injection
50 mg/mL*
For Intramuscular Use Only
10 X 1 mL Single-Dose Vials
Sterile
Rx only
zydus pharmaceuticals
250 mg/5 mL (50 mg/mL) Carton (1 vial per carton)
NDC 72785-0005-1
Haloperidol
Decanoate
Injection
250 mg/5 mL*
(50 mg/mL)
For Intramuscular Use Only
5 mL
Multiple-Dose Vial
Sterile
Rx only
zydus pharmaceuticals
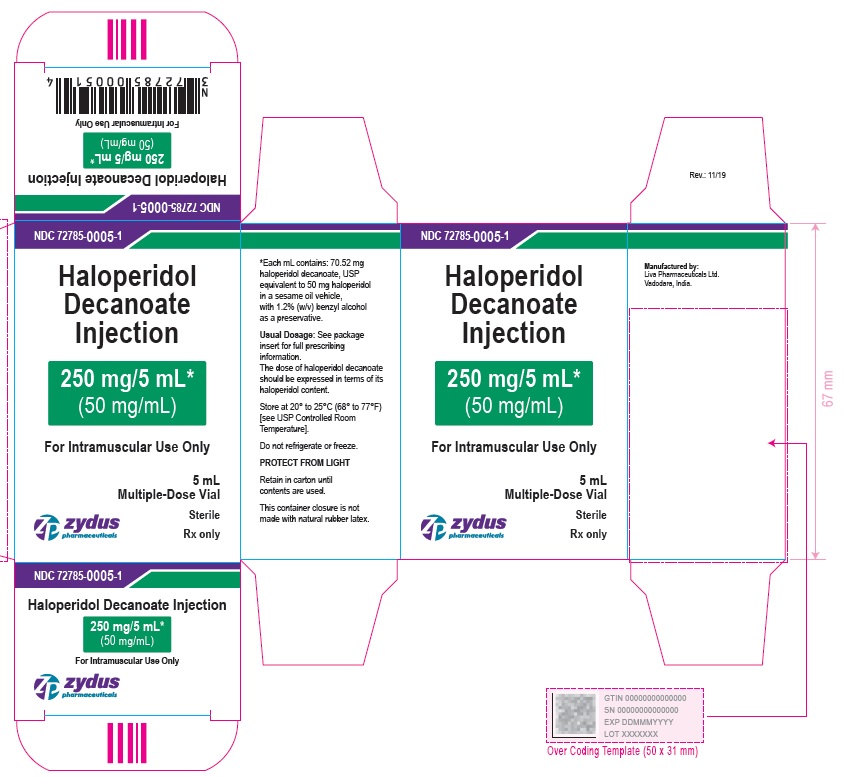
250 mg/5 mL (50 mg/mL) Carton (5 vials per carton)
NDC 72785-0005-5
Haloperidol Decanoate Injection
250 mg/5 mL*
(50 mg/mL)
For Intramuscular Use Only
5 X 5 mL Multiple-Dose Vials
Sterile
Rx only
zydus pharmaceuticals

100 mg/mL Carton (1 vial per carton)
NDC 72785-0006-1
Haloperidol
Decanoate
Injection
100 mg/mL*
For Intramuscular Use Only
1 mL
Single-Dose Vial
Sterile
Rx only
zydus pharmaceuticals
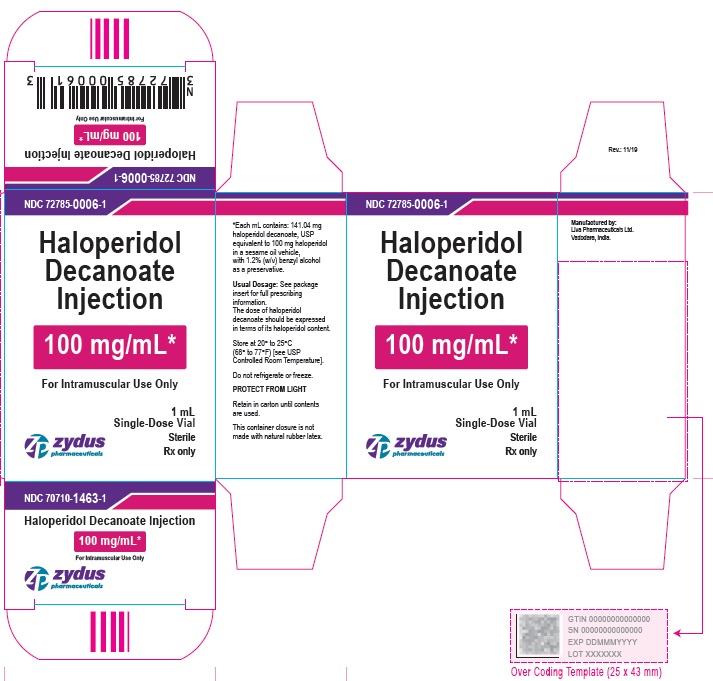
100 mg/mL Carton (5 vials per carton)
NDC 72785-0006-5
Haloperidol Decanoate Injection
100 mg/mL*
For Intramuscular Use Only
5 X 1 mL Single-Dose Vials
Sterile
Rx only
zydus pharmaceuticals
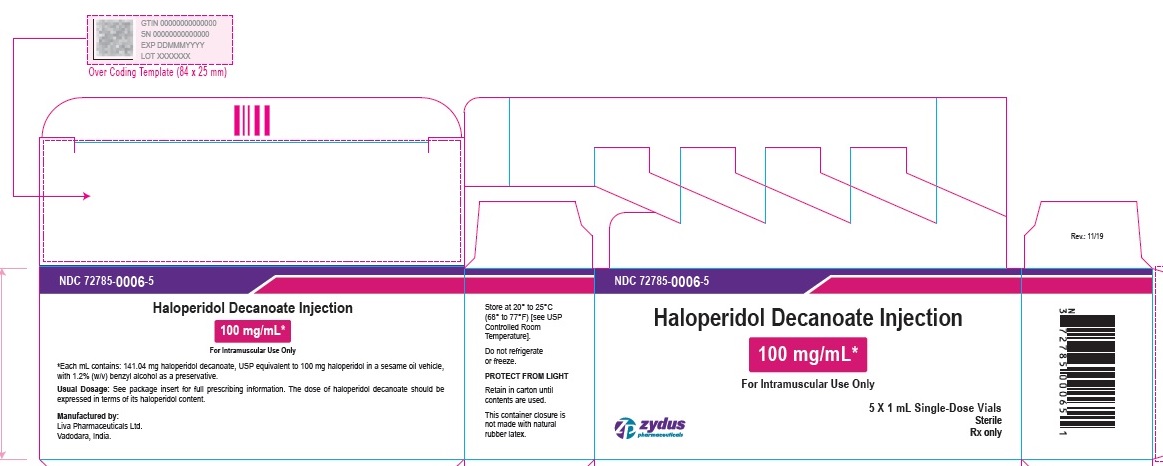
500 mg/5 mL (100 mg/mL) Carton (1 vial per carton)
NDC 72785-0007-1
Haloperidol
Decanoate
Injection
500 mg/5 mL*
(100 mg/mL)
For Intramuscular Use Only
5 mL Multiple-Dose Vial
Sterile
Rx only
zydus pharmaceuticals
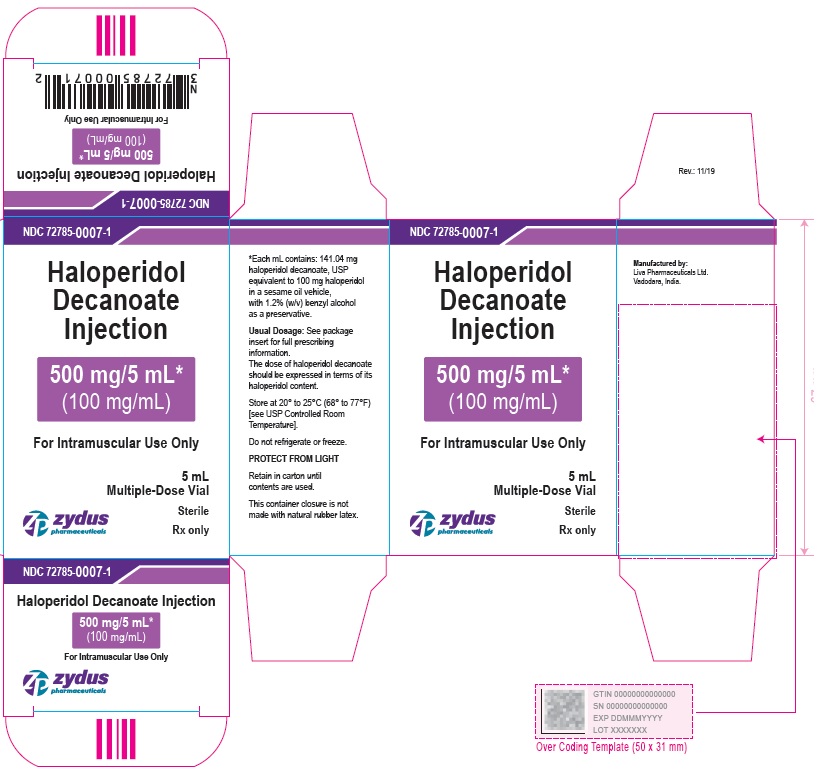
500 mg/5 mL (100 mg/mL) Carton (5 vials per carton)
NDC 72785-0007-5
Haloperidol Decanoate Injection
500 mg/5 mL*
(100 mg/mL)
For Intramuscular Use Only
5 X 5 mL Multiple-Dose Vials
Sterile
Rx only
zydus pharmaceuticals
| HALOPERIDOL DECANOATE
haloperidol decanoate injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| HALOPERIDOL DECANOATE
haloperidol decanoate injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| HALOPERIDOL DECANOATE
haloperidol decanoate injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| HALOPERIDOL DECANOATE
haloperidol decanoate injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Zydus Lifesciences Limited (873671928) |
| Registrant - Zydus Lifesciences Limited (873671928) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zydus Lifesciences Limited | 873671928 | ANALYSIS(72785-0004, 72785-0005, 72785-0006, 72785-0007) , MANUFACTURE(72785-0004, 72785-0005, 72785-0006, 72785-0007) | |