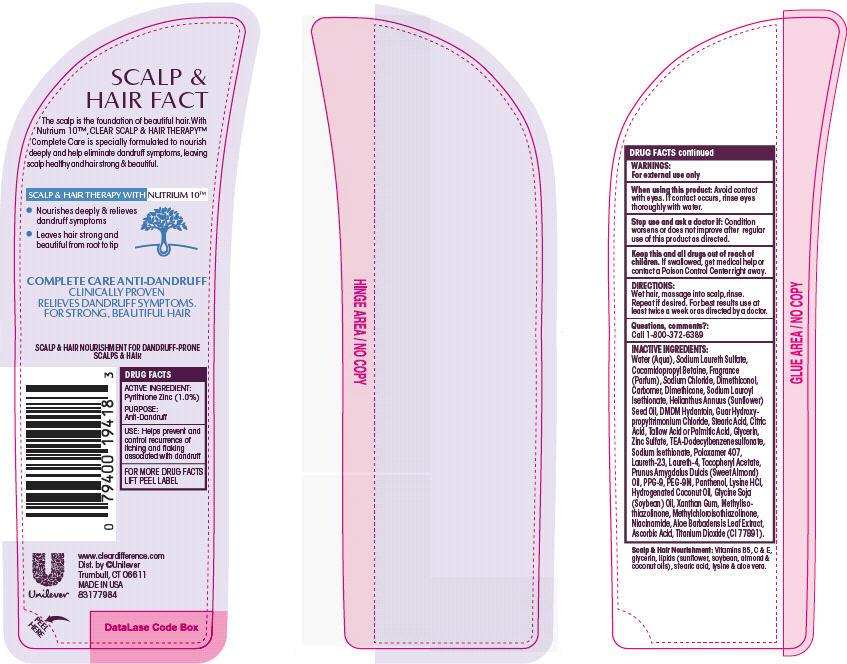
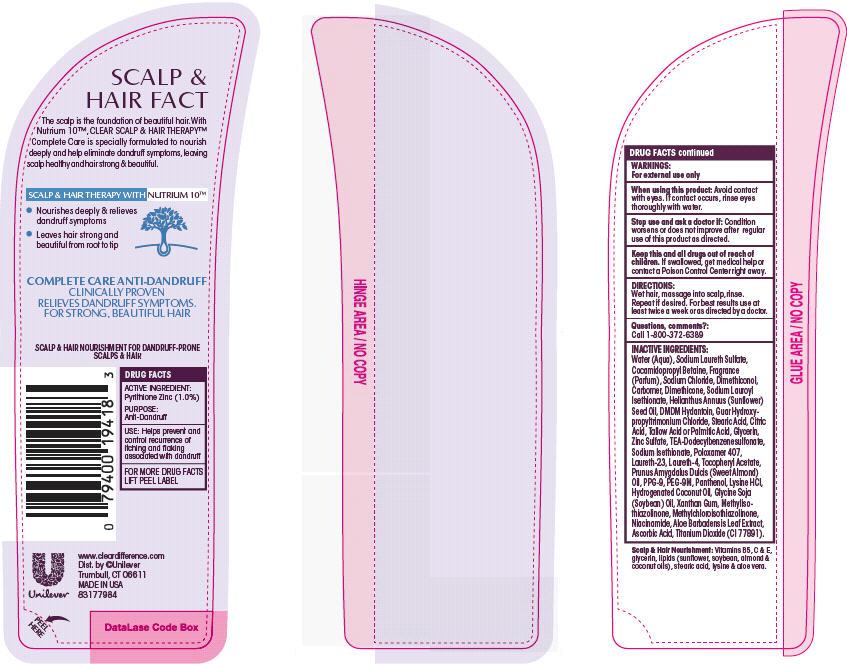
Label: CLEAR COMPLETE CARE ANTIDANDRUFF- pyrithione zinc shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 64942-1252-1, 64942-1252-2, 64942-1252-3 - Packager: Conopco Inc. d/b/a Unilever
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 3, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients
Water (Aqua)
Sodium Laureth Sulfate
Cocamidopropyl Betaine
Fragrance (Parfum)
Sodium Chloride
Dimethiconol
Carbomer
Dimethicone
Sodium Lauroyl Isethionate
Helianthus Annuus (Sunflower) Seed Oil
DMDM Hydantoin
Guar Hydroxypropyltrimonium Chloride
Stearic Acid
Citric Acid
Tallow Acid or Palmitic Acid
Zinc Sulfate
TEA-Dodecylbenzenesulfonate
Sodium Isethionate
Poloxamer 407
Laureth-23
Laureth-4
Tocopheryl Acetate
Prunus Amygdalus Dulcis (Sweet Almond) Oil
PPG-9
PEG-9M
Panthenol
Lysine HCl
Hydrogenated Coconut Oil
Glycine Soja (Soybean) Oil
Xanthan Gum
Methylisothiazolinone
Methylchloroisothiazolinone
Niacinamide
Aloe Barbadensis Leaf Extract
Ascorbic Acid
Titanium Dioxide (CI 77891)
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLEAR COMPLETE CARE ANTIDANDRUFF
pyrithione zinc shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64942-1252 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pyrithione Zinc (UNII: R953O2RHZ5) (Pyrithione - UNII:6GK82EC25D) Pyrithione Zinc 1.0 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAUROYL ISETHIONATE (UNII: M590021Z02) DMDM HYDANTOIN (UNII: BYR0546TOW) TEA-DODECYLBENZENESULFONATE (UNII: 8HM7ZD48HN) SUNFLOWER OIL (UNII: 3W1JG795YI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PPG-9 (UNII: I29VQH0G0B) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) NIACINAMIDE (UNII: 25X51I8RD4) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) STEARIC ACID (UNII: 4ELV7Z65AP) PALMITIC ACID (UNII: 2V16EO95H1) ZINC SULFATE (UNII: 89DS0H96TB) DIMETHICONOL (41 MPA.S) (UNII: 343C7U75XW) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM ISETHIONATE (UNII: 3R36J71C17) POLOXAMER 407 (UNII: TUF2IVW3M2) LAURETH-23 (UNII: N72LMW566G) LAURETH-4 (UNII: 6HQ855798J) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) ALMOND OIL (UNII: 66YXD4DKO9) POLYETHYLENE GLYCOL 400000 (UNII: 439X29GCJZ) PANTHENOL (UNII: WV9CM0O67Z) LYSINE HYDROCHLORIDE (UNII: JNJ23Q2COM) HYDROGENATED COCONUT OIL (UNII: JY81OXM1OM) SOYBEAN OIL (UNII: 241ATL177A) XANTHAN GUM (UNII: TTV12P4NEE) ALOE VERA LEAF (UNII: ZY81Z83H0X) ASCORBIC ACID (UNII: PQ6CK8PD0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64942-1252-1 381 mL in 1 CONTAINER 2 NDC:64942-1252-2 89 mL in 1 CONTAINER 3 NDC:64942-1252-3 50 mL in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 05/09/2012 Labeler - Conopco Inc. d/b/a Unilever (001375088) Establishment Name Address ID/FEI Business Operations Unilever Supply Chain Co. d/b/a Unilever 043510056 manufacture