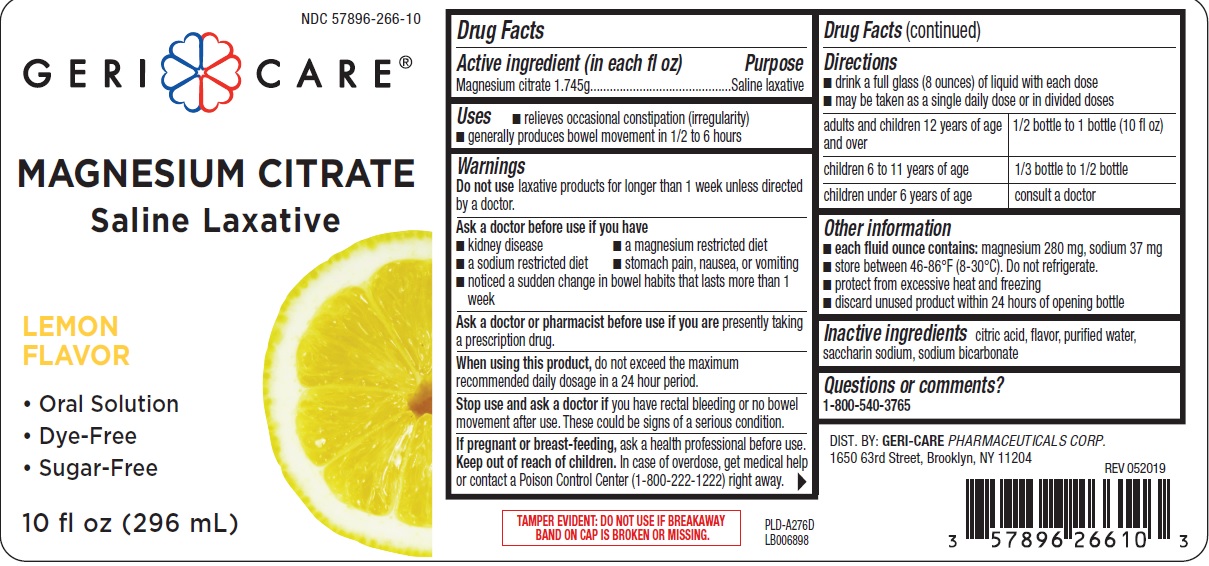
MAGNESIUM CITRATE- magnesium citrate liquid
Geri-Care Pharmaceuticals, Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
qcom
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 1/2 to 6 hours
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium restricted diet
- a sodium restricted diet
- stomach pain, nausea, or vomiting
- noticed a sudden change in bowel habits that lasts more than 1 week
Directions
- drink a full glass (8 ounces) of liquid with each dose
- may be taken as a single daily dose or in divided doses
| adults and children 12 years of age and over | 1/2 bottle to 1 bottle (10 fl oz) |
| children 6 to 11 years of age | 1/3 bottle to 1/2 bottle |
| children under 6 years of age | consult a doctor |
Other information
- each fluid ounce contains: magnesium 280 mg, sodium 37 mg
- store between 46-86ºF (8-30ºC). Do not refrigerate
- protect from excessive heat and freezing
- discard unused product within 24 hours of opening bottle
| MAGNESIUM CITRATE
magnesium citrate liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Geri-Care Pharmaceuticals, Corp (611196254) |
| Registrant - Geri-Care Pharmaceuticals, Corp (611196254) |
Revised: 2/2023
Document Id: f4ac2ae0-9b65-c774-e053-2a95a90a3192
Set id: 99eb67ca-301c-dfdb-e053-2a95a90aff6f
Version: 3
Effective Time: 20230214
Geri-Care Pharmaceuticals, Corp