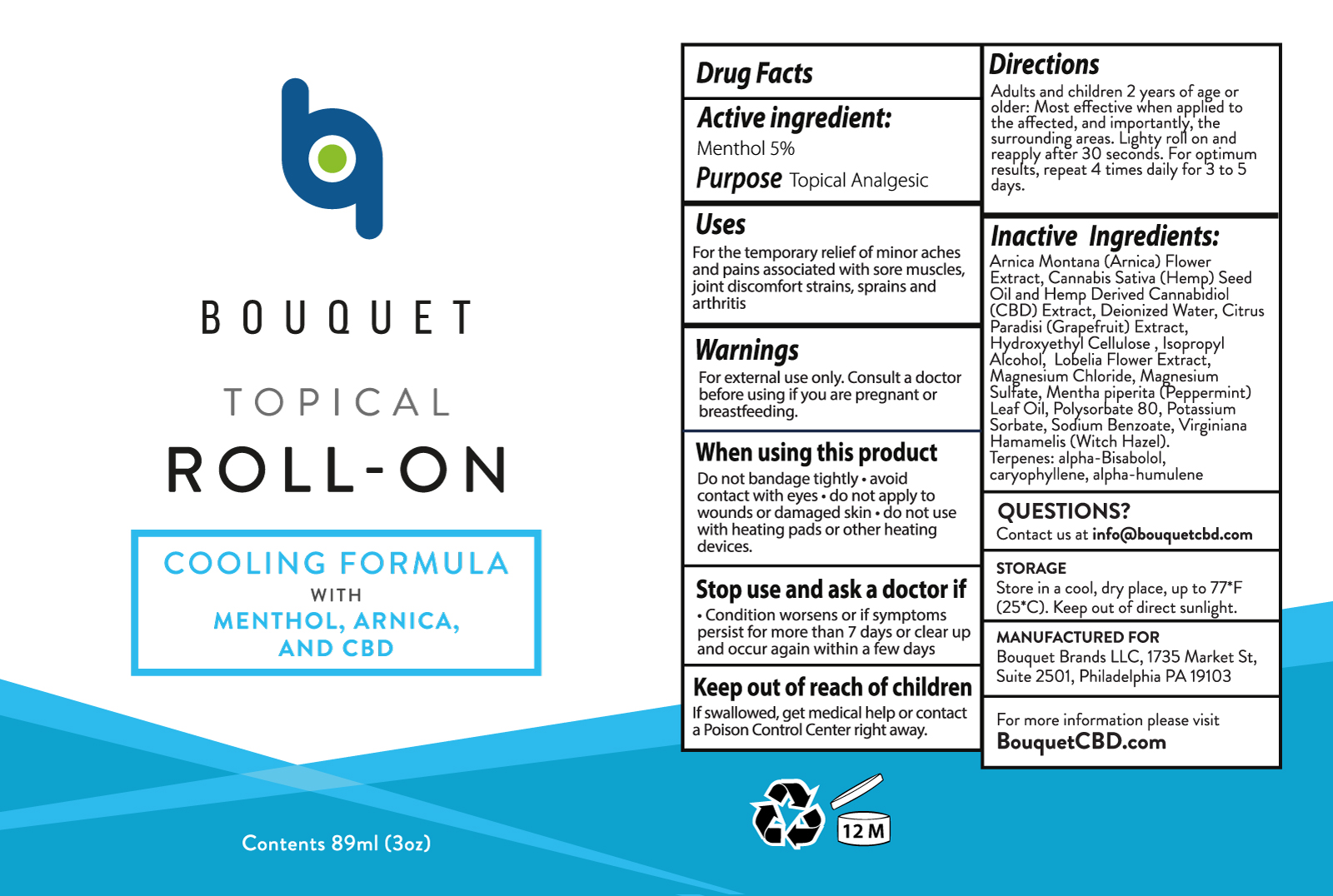
BOUQUET COOLING FORMULA- menthol roll-on liquid
Renu Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BOUQUET ROLL-ON
Uses
For the temporary relief of minor aches and pains associated with sore muscles, joint discomfort, strains, sprains and arthritis
When using this product
- Do not bandage tightly
- avoid contact with eyes
- do not apply to wounds or damaged skin
- do not use with heating pads or other heating devices.
Stop use and ask a doctor if
- Condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 2 years of age or older: Most effective when applied to the affected, and importantly, the surrounding areas. Light roll on and reapply after 30 seconds. For optimum results, repeat 4 times daily for 3 to 5 days.
Inactive Ingredients:
Arnica Montana (Arnica) Flower Extract, Cannabis Sativa (Hemp) Seed Oil and Hemp Derived Cannabidiol (CBD) Extract, Deionized Water, Citrus Paradisi (Grapefruit) Extract, Hydroxyethyl Cellulose, Isopropyl Alcohol, Lobelia Flower Extract, Magnesium Chloride, Magnesium Sulfate, Mentha piperita (Peppermint) Leaf Oil, Polysorbate 80, Potassium Sorbate, Sodium Benzoate, Virginiana Hamamelis (Witch Hazel), Terpenes: alpha-Bisabolol, caryophyllene, alpha-humulene
| BOUQUET COOLING FORMULA
menthol roll-on liquid |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Renu Laboratories, Inc. (945739449) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Renu Laboratories, Inc. | 945739449 | manufacture(76348-460) | |