RAPID MELTS CHILDRENS- acetaminophen tablet, chewable
CHAIN DRUG MARKETING ASSOCIATION INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Quality Choice 44-452
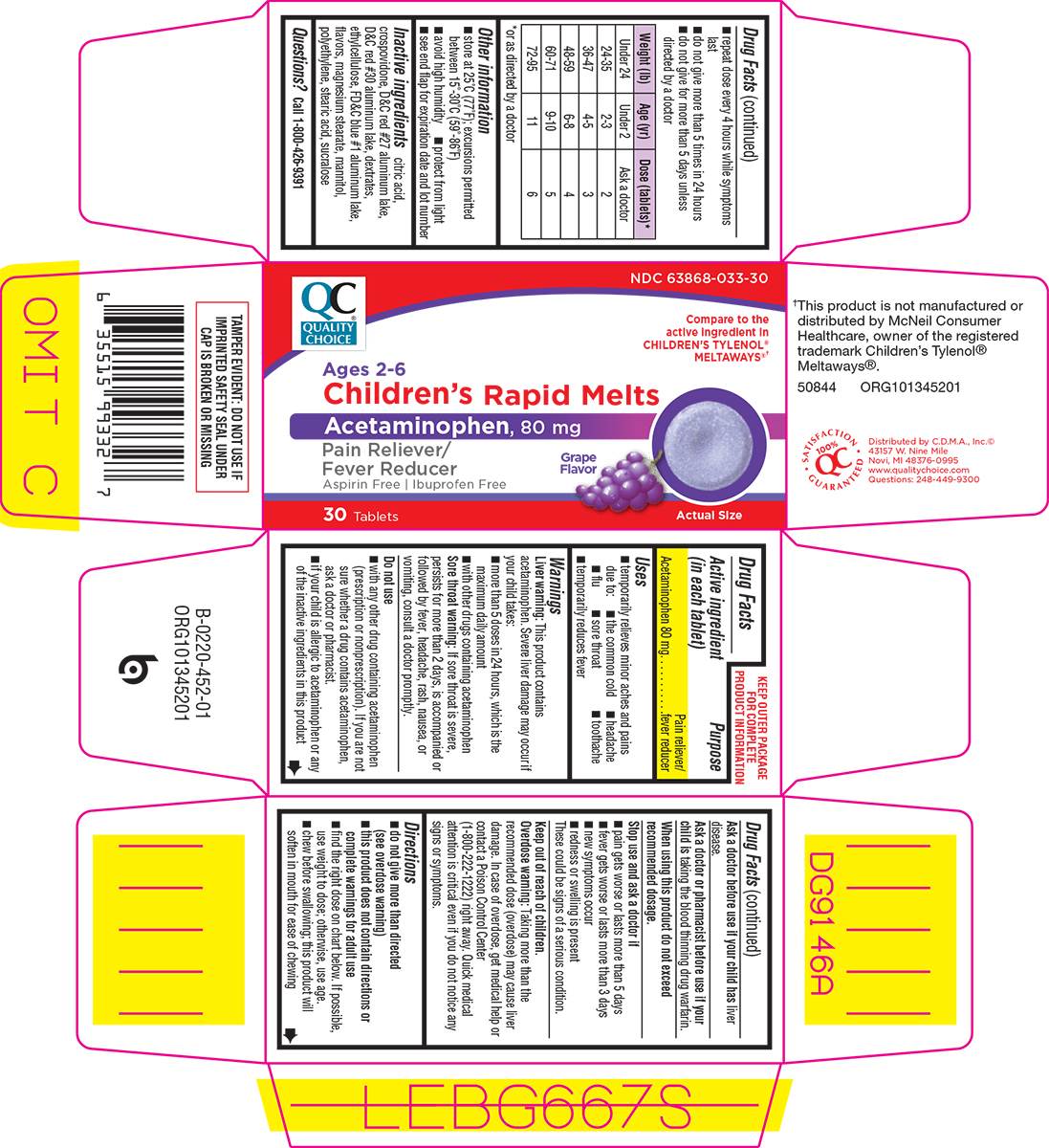
Uses
- temporarily relieves minor aches and pains due to:
- the common cold
- headache
- flu
- sore throat
- toothache
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes:
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
Stop use and ask a doctor if
- pain gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical even if you do not notice any signs or symptoms.
Directions
- do not give more than directed (see overdose warning)
- this product does not contain directions or complete warnings for adult use
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew before swallowing; this product will soften in mouth for ease of chewing
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
|
Weight (lb) |
Age (yr) |
Dose (tablets)* |
|
Under 24 |
Under 2 |
Ask a doctor |
|
24-35 |
2-3 |
2 |
|
36-47 |
4-5 |
3 |
|
48-59 |
6-8 |
4 |
|
60-71 |
9-10 |
5 |
|
72-95 |
11 |
6 |
*or as directed by a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- avoid high humidity
- protect from light
- see end flap for expiration date and lot number
Inactive ingredients
citric acid, crospovidone, D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrates, ethylcellulose, FD&C blue #1 aluminum lake, flavors, magnesium stearate, mannitol, polyethylene, stearic acid, sucralose
Principal Display Panel
QC®
QUALITY
CHOICE
NDC 63868-033-30
Compare to the
active ingredient in
CHILDREN’S TYLENOL®
MELTAWAYS®†
Ages 2-6
Children’s Rapid Melts
Acetaminophen, 80 mg
Pain Reliever/
Fever Reducer
Aspirin Free | Ibuprofen Free
Grape
Flavor
30 Tablets
Actual Size
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER
CAP IS BROKEN OR MISSING
†This product is not manufactured or
distributed by McNeil Consumer
Healthcare, owner of the registered
trademark Children’s Tylenol®
Meltaways®.
50844 ORG101345201
SATISFACTION
GUARANTEED
100%
QC
Distributed by C.D.M.A., Inc.©
43157 W. Nine Mile
Novi, MI 48376-0995
www.qualitychoice.com
Questions: 248-449-9300
Quality Choice 44-452
| RAPID MELTS
CHILDRENS
acetaminophen tablet, chewable |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - CHAIN DRUG MARKETING ASSOCIATION INC (011920774) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(63868-033) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(63868-033) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | PACK(63868-033) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | PACK(63868-033) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | PACK(63868-033) | |