CUTIVATE- fluticasone propionate lotion
PharmaDerm a division of Fougera Pharmaceuticals Inc.
----------
CUTIVATE®
(fluticasone propionate)
Lotion, 0.05%
DESCRIPTION
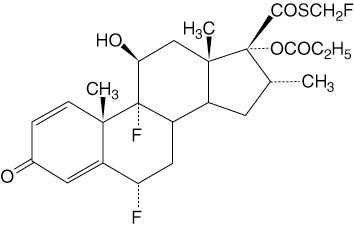
CUTIVATE® (fluticasone propionate) Lotion, 0.05% contains fluticasone propionate [S-(fluoromethyl)6α,9-difluoro-11β,17-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioate, 17-propionate], a synthetic fluorinated corticosteroid, for topical dermatologic use. The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Chemically, fluticasone propionate is C25H31F3O5S. It has the following structural formula:
Fluticasone propionate is a white to off-white powder with a molecular weight of 500.6. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
Each gram of CUTIVATE® Lotion contains 0.5mg fluticasone propionate in a base of cetostearyl alcohol, isopropyl myristate, propylene glycol, cetomacrogol 1000, dimethicone 360, citric acid, sodium citrate, and purified water, with imidurea, methylparaben, and propylparaben as preservatives.
CLINICAL PHARMACOLOGY
Like other topical corticosteroids, fluticasone propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid.
Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Although fluticasone propionate has a weak affinity for the progesterone receptor and virtually no affinity for the mineralocorticoid, estrogen or androgen receptors, the clinical relevance as related to safety is unknown. Fluticasone propionate is lipophilic and has strong affinity for the glucocorticoid receptor. The therapeutic potency of glucocorticoids is related to the half-life of the glucocorticoid receptor complex. The half-life of the fluticasone propionate-glucocorticoid receptor complex is approximately 10 hours.
Pharmacokinetics
Absorption: The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressing enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption.
Distribution: Following intravenous administration of 1 mg of fluticasone propionate in healthy volunteers, the initial disposition phase for fluticasone propionate was rapid and consistent with its high lipid solubility and tissue binding. The apparent volume of distribution averaged 4.2 L/kg (range, 2.3 to 16.7 L/kg). The percentage of fluticasone propionate bound to human plasma proteins averaged 91%. Fluticasone propionate is weakly and reversibly bound to erythrocytes. Fluticasone propionate is not significantly bound to human transcortin.
Metabolism: No metabolites of fluticasone propionate were detected in an in vitro study of radiolabeled fluticasone propionate incubated in a human skin homogenate. The total blood clearance of systemically absorbed fluticasone propionate averages 1093 mL/min (range, 618 to 1702 mL/min) after a 1-mg intravenous dose, with renal clearance accounting for less than 0.02% of the total.
Orally absorbed fluticasone propionate has demonstrated extensive first-pass metabolism with no unchanged drug detected in the plasma up to 6 hours after dosing. Fluticasone propionate is metabolized in the liver by cytochrome P450 3A4-mediated hydrolysis of the 5-fluoromethyl carbothiolate grouping. This transformation occurs in 1 metabolic step to produce the inactive 17β-carboxylic acid metabolite, the only known metabolite detected in man. This metabolite has approximately 2000 times less affinity than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
Excretion: Following an intravenous dose of 1 mg in healthy volunteers, fluticasone propionate showed polyexponential kinetics and had an average terminal half-life of 7.2 hours (range, 3.2 to 11.2 hours).
Special Population (Pediatric): Plasma fluticasone levels were measured in patients 2 years - 6 years of age in an HPA axis suppression study. A total of 13 (62%) of 21 patients tested had measurable fluticasone at the end of 3 - 4 weeks of treatment. The mean ± SD fluticasone plasma values for patients aged under 3 years was 47.7 ± 31.7 pg/mL and 175.5 ± 243.6 pg/mL. Three patients had fluticasone levels over 300 pg/mL, with one of these having a level of 819.81 pg/mL. No data was obtained for patients < 2 years of age.
CLINICAL STUDIES
CUTIVATE® Lotion applied once daily was superior to vehicle in the treatment of atopic dermatitis in two studies. The two studies enrolled 438 patients with atopic dermatitis aged 3 months and older, of which 169 patients were selected as having clinically significant* signs of erythema, infiltration/papulation and erosion/oozing/crusting at baseline. Table 1 presents the percentage of patients who completely cleared of erythema, infiltration/papulation and erosion/oozing/crusting at Week 4 out of those patients with clinically significant baseline signs.
| *Clinically significant was defined as having moderate or severe involvement for at least two of the three signs (erythema, infiltration/papulation, or erosion/oozing/crusting) in at least 2 body regions. Patients who had moderate to severe disease in a single body region were excluded from the analysis. | ||
|
CUTIVATE® Lotion |
Vehicle |
|
|
Study 1 |
9/45 (20%) |
0/37 (0%) |
|
Study 2 |
7/44 (16%) |
1/43 (2%) |
INDICATIONS AND USAGE
CUTIVATE® (fluticasone propionate) Lotion is indicated for the relief of the inflammatory and pruritic manifestations of atopic dermatitis in patients 1 year of age or older. The safety and efficacy of drug use for longer than 4 weeks in this population have not been established. The safety and efficacy of CUTIVATE® Lotion in pediatric patients below 1 year of age have not been established.
CONTRAINDICATIONS
CUTIVATE® Lotion is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation (see PRECAUTIONS).
PRECAUTIONS
CUTIVATE® Lotion contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. CUTIVATE® Lotion should not be used in individuals with hypersensitivity to formaldehyde as it may prevent healing or worsen dermatitis.
General: Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal from treatment. Manifestations of Cushing's syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment.
Patients applying a potent topical steroid to a large surface area or to areas under occlusion should be evaluated periodically for evidence of HPA axis suppression. This may be done by using cosyntropin (ACTH1·24) stimulation testing.
Forty-two pediatric patients (4 months to < 6 years of age) with moderate to severe atopic eczema who were treated with CUTIVATE® Lotion for at least 3-4 weeks were assessed for HPA axis suppression and 40 of these subjects applied at least 90% of applications. None of the 40 evaluable patients suppressed, where the sole criterion for HPA axis suppression is a plasma cortisol level of less than or equal to 18 micrograms per deciliter after cosyntropin stimulation. Although HPA axis suppression was observed in 0 of 40 pediatric patients (upper 95% confidence bound is 7.2%), the occurrence of HPA axis suppression in any patient and especially with longer use cannot be ruled out. In other studies with fluticasone propionate topical formulations, adrenal suppression has been observed.
If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products.
Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios (see PRECAUTIONS: Pediatric Use).
The following local adverse reactions have been reported with topical corticosteroids, and they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions are listed in an approximately decreasing order of occurrence: irritation, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, hypertrichosis, and miliaria.
CUTIVATE Lotion, 0.05% may cause local cutaneous adverse reactions (see ADVERSE REACTIONS).
If irritation develops, CUTIVATE® Lotion should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of CUTIVATE® Lotion should be discontinued until the infection has been adequately controlled.
CUTIVATE® Lotion should not be used in the presence of preexisting skin atrophy and should not be used where infection is present at the treatment site. CUTIVATE® Lotion should not be used in the treatment of rosacea and perioral dermatitis.
Patients that apply CUTIVATE® Lotion to exposed portions of the body should avoid excessive exposure to either natural or artificial sunlight (including tanning booths, sun lamps, etc.).
Information for Patients: Patients using CUTIVATE® Lotion should receive the following information and instructions:
- 1.
- CUTIVATE® Lotion is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- 2.
- CUTIVATE® Lotion should not be used for any disorder other than that for which it was prescribed.
- 3.
- The treated skin area should not be bandaged or otherwise covered or wrapped so as to be occlusive unless directed by the physician.
- 4.
- Patients should report to their physician any signs of local adverse reactions as well as non-healing or worsening of skin condition.
- 5.
- Parents of pediatric patients should be advised not to use this medication in the treatment of diaper dermatitis unless directed by the physician. CUTIVATE® Lotion should not be applied in the diaper areas as diapers or plastic pants may constitute occlusive dressing (see DOSAGE AND ADMINISTRATION).
- 6.
- CUTIVATE® Lotion should not be used on the face, underarms, or groin areas unless directed by a physician.
- 7.
- CUTIVATE® Lotion therapy should be discontinued if control is achieved before 4 weeks. If no improvement is seen within 2 weeks, contact a physician. The safety of the use of CUTIVATE® Lotion for longer than 4 weeks has not been established.
- 8.
- Patients should report to their physician if they are allergic to formaldehyde.
- 9.
- Patients that apply CUTIVATE® Lotion to exposed portions of the body should follow physician advice and routine precautions to avoid excessive or unnecessary exposure to either natural or artificial sunlight (such as sunbathing, tanning booths, sun lamps, etc.).
Laboratory Tests: The cosyntropin (ACTH1·24) stimulation test may be helpful in evaluating patients for HPA axis suppression.
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
In an oral (gavage) mouse carcinogenicity study, doses of 0.1, 0.3 and 1 mg/kg/day fluticasone propionate were administered to mice for 18 months. Fluticasone propionate demonstrated no tumorigenic potential at oral doses up to 1 mg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
In a dermal mouse carcinogenicity study, 0.05% fluticasone propionate ointment (40 μl) was topically administered for 1, 3 or 7 days/week for 80 weeks. Fluticasone propionate demonstrated no tumorigenic potential at dermal doses up to 6.7 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
In a 52 week dermal photo-carcinogenicity study conducted in hairless albino mice with concurrent exposure to low level ultraviolet radiation (40 weeks of treatment followed by 12 weeks of observation), topically treated lotion vehicle animals and 0.05% fluticasone propionate lotion animals demonstrated shorter time to benign skin tumor formation compared to untreated control animals. Lotion vehicle treated animals developed benign skin tumors in a shorter time than 0.05% fluticasone propionate lotion treated animals. Although applicability of results to clinical use of CUTIVATE® Lotion in humans is unknown, and enhanced tumor growth in patients treated with CUTIVATE® Lotion has not been reported, patients should exercise general precautions in minimizing UV light exposure by avoiding excessive or unnecessary exposure to either natural or artificial sunlight (including sunbathing, tanning booths, sun lamps, etc.)
Fluticasone propionate revealed no evidence of mutagenic or clastogenic potential based on the results of five in vitro genotoxicity tests (Ames assay, E. coli fluctuation test, S. cerevisiae gene conversion test, Chinese hamster ovary cell chromosome aberration assay and human lymphocyte chromosome aberration assay) and one in vivo genotoxicity test (mouse micronucleus assay).
No evidence of impairment of fertility or effect on mating performance was observed in a fertility and general reproductive performance study conducted in male and female rats at subcutaneous doses up to 50 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Pregnancy
Teratogenic Effects: Pregnancy Category C. Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Systemic embryofetal development studies were conducted in mice, rats and rabbits. Subcutaneous doses of 15, 45 and 150 μg/kg/day of fluticasone propionate were administered to pregnant female mice from gestation days 6 – 15. A teratogenic effect characteristic of corticosteroids (cleft palate) was noted after administration of 45 and 150 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 15 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 10, 30 and 100 μg/kg/day of fluticasone propionate were administered to pregnant female rats in two embryofetal development studies (one study administered fluticasone propionate from gestation days 6 – 15 and the other study from gestation days 7 – 17). In the presence of maternal toxicity, fetal effects noted at 100 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, omphalocele, cleft palate, and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 10 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 0.08, 0.57 and 4 μg/kg/day of fluticasone propionate were administered to pregnant female rabbits from gestation days 6 – 18. Fetal effects noted at 4 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, cleft palate and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 0.57 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Oral doses of 3, 30 and 300 μg/kg/day fluticasone propionate were administered to pregnant female rabbits from gestation days 8 – 20. No fetal or teratogenic effects were noted at oral doses up to 300 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. However, no fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration (see CLINICAL PHARMACOLOGY).
Fluticasone propionate crossed the placenta following administration of a subcutaneous or an oral dose of 100 μg/kg tritiated fluticasone propionate to pregnant rats.
There are no adequate and well-controlled studies in pregnant women. During clinical trials of CUTIVATE® Lotion, women of childbearing potential were required to use contraception to avoid pregnancy. Therefore, CUTIVATE® Lotion should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when CUTIVATE® Lotion is administered to a nursing woman.
Pediatric Use: CUTIVATE® Lotion contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. CUTIVATE® Lotion should not be used in individuals with hypersensitivity to formaldehyde as it may prevent healing or worsen dermatitis.
CUTIVATE® Lotion should be discontinued if control is achieved before 4 weeks. If no improvement is seen within 2 weeks, contact a physician. The safety of the use of CUTIVATE® Lotion for longer than 4 weeks has not been established.
The safety and efficacy of CUTIVATE® Lotion in pediatric patients below 1 year of age have not been established.
Parents of pediatric patients should be advised not to use this medication in the treatment of diaper dermatitis unless directed by the physician. CUTIVATE® Lotion should not be applied in the diaper areas as diapers or plastic pants may constitute occlusive dressing.
Forty-two pediatric patients (4 months to < 6 years of age) with moderate to severe atopic eczema who were treated with CUTIVATE® Lotion for at least 3-4 weeks were assessed for HPA axis suppression and 40 of these subjects applied at least 90% of applications. None of the 40 evaluable patients suppressed, where the sole criterion for HPA axis suppression is a plasma cortisol level of less than or equal to 18 micrograms per deciliter after cosyntropin stimulation. Although HPA axis suppression was observed in 0 of 40 pediatric patients (upper 95% confidence bound is 7.2%), the occurrence of HPA axis suppression in any patient and especially with longer use cannot be ruled out.
In other studies with fluticasone propionate topical formulations, adrenal suppression has been observed. CUTIVATE® (fluticasone propionate) Cream, 0.05% caused HPA axis suppression in 2 of 43 pediatric patients, ages 2 and 5 years old, who were treated for 4 weeks covering at least 35% of the body surface area. Follow-up testing 12 days after treatment discontinuation, available for 1 of the 2 patients, demonstrated a normally responsive HPA axis.
HPA axis suppression, Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include low plasma cortisol levels to an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema. Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
In addition, local adverse events including cutaneous atrophy, striae, telangiectasia, and pigmentation change have been reported with topical use of corticosteroids in pediatric patients.
Geriatric Use: A limited number of patients above 65 years of age have been treated with CUTIVATE® Lotion in US and non-US clinical trials. Specifically only 8 patients above 65 years of age were treated with CUTIVATE® Lotion in controlled clinical trials. The number of patients is too small to permit separate analyses of efficacy and safety.
ADVERSE REACTIONS
Clinical Trial Experience: In 2 multicenter vehicle-controlled clinical trials of once-daily application of CUTIVATE® Lotion by 196 adult and 242 pediatric patients, the total incidence of adverse reactions considered drug related by investigators was approximately 4%. Events were local cutaneous events, usually mild and self-limiting, and consisted primarily of burning/stinging (2%). All other drug-related events occurred with an incidence of less than 1%, and inclusively were contact dermatitis, exacerbation of atopic dermatitis, folliculitis of legs, pruritus, pustules on arm, rash, and skin infection.
The incidence of drug-related events on drug compared to vehicle (4% and 5%, respectively) was similar. The incidence of drug-related events between study populations of 242 pediatric patients (age 3 months to < 17 years) and 196 adult patients (17 years or older) (4% and 5%, respectively) was also similar.
In an open-label study of 44 pediatric patients applying CUTIVATE® Lotion to at least 35% of body surface area twice daily for 3 or 4 weeks, the overall incidence of drug-related adverse events was 14%. Events were local, cutaneous, and inclusively were dry skin (7%), stinging at application site (5%), and excoriation (2%).
|
Adverse Events |
CUTIVATE® Lotion |
Vehicle |
|
N=221 |
N=217 |
|
|
Burning/Stinging skin |
4 (2%) |
3 (1%) |
|
Contact Dermatitis |
0 |
1 (<1%) |
|
Exacerbation of Atopic dermatitis |
0 |
1 (<1%) |
|
Folliculitis of legs |
2 (<1%) |
0 |
|
Irritant Contact Dermatitis |
0 |
1 (<1%) |
|
Pruritus |
1 (<1%) |
1 (<1%) |
|
Pustules on Arms |
1 (<1%) |
0 |
|
Rash |
1 (<1%) |
2 (<1%) |
|
Skin Infection |
0 |
3 (1%) |
|
Adverse Events |
CUTIVATE® Lotion Twice Daily |
|
Dry skin at multiple sites |
3 (7%) |
|
Stinging at Application Sites |
2 (5%) |
|
Excoriation |
1 (2%) |
The table below summarizes all adverse events by body system that occurred in at least 1% of patients in either the drug or vehicle group in controlled clinical trials.
|
Body System |
CUTIVATE® Lotion |
Vehicle Lotion |
|
N = 221 |
N = 217 |
|
|
Any Adverse Event |
77 (35%) |
82 (38%) |
|
Skin | ||
|
Burning and Stinging |
4 (2%) |
3 (1%) |
|
Pruritus |
3 (1%) |
5 (2%) |
|
Rash |
2 (<1%) |
3 (1%) |
|
Skin Infection |
0 |
3 (1%) |
|
Ear, Nose, Throat | ||
|
Common Cold |
9 (4%) |
5 (2%) |
|
Ear Infection |
3 (1%) |
3 (1%) |
|
Nasal Sinus Infection |
2 (<1%) |
4 (2%) |
|
Rhinitis |
1 (<1%) |
3 (1%) |
|
Upper Respiratory |
6 (3%) |
7 (3%) |
|
Gastrointestinal | ||
|
Normal Tooth Eruption |
2 (< 1%) |
3 (1%) |
|
Diarrhea |
3 (1%) |
0 |
|
Vomiting |
3 (1%) |
2 (<1%) |
|
Lower Respiratory | ||
|
Cough |
7 (3%) |
6 (3%) |
|
Influenza |
5 (2%) |
0 |
|
Wheeze |
0 |
3 (1%) |
|
Neurology | ||
|
Headache |
4 (2%) |
5 (2%) |
|
Non-Site Specific | ||
|
Fever |
8 (4%) |
8 (4%) |
|
Seasonal Allergy |
2 (<1%) |
3 (1%) |
During the clinical trials, eczema herpeticum occurred in a 33-year-old male patient treated with CUTIVATE® Lotion. Additionally, a 4-month-old patient treated with CUTIVATE® Lotion in the open-label trial had marked elevations of the hepatic enzymes AST and ALT.
Post Marketing Experience: Systemic adverse events with CUTIVATE® Cream and CUTIVATE® Ointment have included: immunosuppression/Pneumocystis carinii pneumonia/leukopenia/thrombocytopenia; hyperglycemia/ glycosuria; Cushing syndrome; generalized body edema/blurred vision; and acute urticarial reaction (edema, urticaria, pruritus, and throat swelling).
The following localized adverse reactions have been reported during post approval use of CUTIVATE® Lotion: erythema, edema/swelling, bleeding, and a reported lack of efficacy.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
OVERDOSAGE
Topically applied CUTIVATE® Lotion can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS).
DOSAGE AND ADMINISTRATION
CUTIVATE® Lotion may be used in adult and pediatric patients 1 year of age or older. The safety and efficacy of CUTIVATE® Lotion in pediatric patients below 1 year of age have not been established (see PRECAUTIONS: Pediatric Use).
Atopic Dermatitis: Apply a thin film of CUTIVATE® Lotion to the affected skin areas once daily. Rub in gently.
As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. The safety and efficacy of drug use for longer than 4 weeks have not been established.
CUTIVATE® Lotion should not be used with occlusive dressings or applied in the diaper area unless directed by a physician.
HOW SUPPLIED
CUTIVATE® Lotion is supplied in
60 mL bottle NDC 0462-0434-60
120 mL bottle NDC 0462-0434-04
Store between 15° and 30°C (59° and 86°F). Do not refrigerate.
Keep container tightly sealed.
Revised: 05/2011
PharmaDerm®
A division of Nycomed US Inc.
Melville, NY 11747 USA
www.pharmaderm.com
Patent No. 7300669
I8434
| CUTIVATE
fluticasone propionate lotion |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - PharmaDerm a division of Fougera Pharmaceuticals Inc. (043838424) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Whitehouse Analytical Laboratories, LLC | 138628008 | ANALYSIS(0462-0434) | |