PHYSOSTIGMINE SALICYLATE- physostigmine salicylate injection
General Injectables and Vaccines, Inc.a
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
PHYSOSTIGMINE SALICYLATE INJECTION
Rx only
DESCRIPTION
Physostigmine Salicylate Injection is a derivative of the Calabar bean, and its active moiety, physostigmine, is also known as eserine. Its chemical structure is:
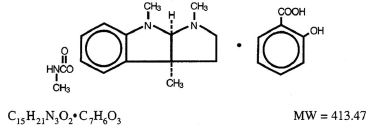
It is soluble in water and a 0.5% aqueous solution has a pH of 5.8.
Physostigmine Salicylate Injection is available in 2 mL ampules, each mL containing 1 mg of Physostigmine Salicylate in a vehicle composed of sodium metabisulfite 0.1%, benzyl alcohol 2.0% as a preservative in Water for Injection.
CLINICAL PHARMACOLOGY
Physostigmine Salicylate Injection is a reversible anticholinesterase which effectively increases the concentration of acetylcholine at the sites of cholinergic transmission. The action of acetylcholine is normally very transient because of its hydrolysis by the enzyme, acetylcholinesterase. Physostigmine Salicylate Injection inhibits the destructive action of acetylcholinesterase and thereby prolongs and exaggerates the effect of the acetylcholine.
Physostigmine Salicylate Injection contains a tertiary amine and easily penetrates the blood brain barrier, while an anticholinesterase, such as neostigmine, which has a quaternary ammonium ion is not capable of crossing the barrier. Physostigmine Salicylate Injection can reverse both central and peripheral anticholinergia. The anticholinergic syndrome has both central and peripheral signs and symptoms. Central toxic effects include anxiety, delirium, disorientation, hallucinations, hyperactivity and seizures. Severe poisoning may produce coma, medullary paralysis and death. Peripheral toxicity is characterized by tachycardia, hyperpyrexia, mydriasis, vasodilation, urinary retention, diminution of gastrointestinal motility, decrease of secretion in salivary and sweat glands, and loss of secretions in the pharynx, bronchi, and nasal passages.
Dramatic reversal of the effects of anticholinergic symptoms can be expected in minutes after the intravenous administration of Physostigmine Salicylate Injection, if the diagnosis is correct and the patient has not suffered anoxia or other insult. The duration of action of Physostigmine Salicylate Injection is relatively short, approximately 45 to 60 minutes.
Numerous drugs and some plants produce the anticholinergic syndrome either directly or as a side effect; this undesirable or potentially dangerous phenomenon may be brought about by either therapeutic doses or overdoses of the drugs. Such drugs include among others, atropine, other derivatives of the belladonna alkaloids, tricyclic antidepressants, phenothiazines, and antihistamines.
INDICATIONS AND USAGE
To reverse the effect upon the central nervous system, caused by clinical or toxic dosages of drugs capable of producing the anticholinergic syndrome.
CONTRAINDICATIONS
Physostigmine Salicylate Injection should not be used in the presence of asthma, gangrene, diabetes, cardiovascular disease, mechanical obstruction of the intestine or urogenital tract or any vagotonic state, and in patients receiving choline esters and depolarizing neuromuscular blocking agents (decamethonium, succinylcholine).
For post-anesthesia, the concomitant use of atropine with physostigmine salicylate is not recommended, since the atropine antagonizes the action of physostigmine.
WARNINGS
Contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
If excessive symptoms of salivation, emesis, urination and defecation occur, the use of Physostigmine Salicylate Injection should be terminated. If excessive sweating or nausea occur, the dosage should be reduced.
Intravenous administration should be at a slow, controlled rate, no more than 1 mg per minute (see DOSAGE). Rapid administration can cause bradycardia, hypersalivation leading to a respiratory difficulties and possible convulsions.
An overdosage of Physostigmine Salicylate Injection can cause a cholinergic crisis.
PRECAUTIONS
Because of the possibility of hypersensitivity in an occasional patient, atropine sulfate injection should always be at hand since it is an antagonist and antidote for physostigmine.
USAGE IN PREGNANCY
Safe use in pregnancy and lactation has not been established; therefore, use in pregnant women, nursing mothers or women who may become pregnant requires that possible benefits be weighed against possible hazards to mother and child.
ADVERSE REACTIONS
Nausea, vomiting and salivation; can be offset by reducing dosage. Bradycardia and convulsions, if intravenous administration is too rapid. See DOSAGE AND ADMINISTRATION.
DOSAGE AND ADMINISTRATION
Post Anesthesia Care: 0.5 to 1.0 mg intramuscularly or intravenously. INTRAVENOUS ADMINISTRATION SHOULD BE AT A SLOW CONTROLLED RATE OF NO MORE THAN 1 MG PER MINUTE. Dosage may be repeated at intervals of 10 to 30 minutes if desired patient response is not obtained.
OVERDOSAGE OF DRUGS THAT CAUSE ANTICHOLINERGIA
2.0 mg intramuscularly or INTRAVENOUSLY AT SLOW CONTROLLED RATE (SEE ABOVE). Dosage may be repeated if life threatening signs, such as arrhythmia, convulsions or coma occurs.
PEDIATRIC DOSAGE
Recommended dosage is 0.02 mg/kg, intramuscularly or by slow intravenous injection, no more than 0.5 mg per minute. If the toxic effects persist, and there is no sign of cholinergic effects, the dosage may be repeated at 5 to 10 minute intervals until a therapeutic effect is obtained or a maximum of 2 mg dosage is attained.
IN ALL CASES OF POISONING, THE USUAL SUPPORTIVE MEASURES SHOULD BE UNDERTAKEN.
STORAGE
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
SOME DRUGS WHICH PRODUCE THE ANTICHOLINERGIC SYNDROME
Amitriptyline, Amoxapine, Anisotropine, Atropine, Benztropine, Biperiden, Carbinoxamine, Clidinium, Cyclobenzaprine, Desipramine, Doxepin, Homatropine, Hyoscine, Hyoscyamine, Hyoscyamus, Imipramine, Lorazepam, Maprotiline, Mepenzolate, Nortriptyline, Propantheline, Protriptyline, Scopolamine, Trimipramine.
SOME PLANTS THAT PRODUCE THE ANTICHOLINERGIC SYNDROME
Black Henbane, Deadly Night Shade, Devil's Apple, Jimson Weed, Loco Seeds or Weeds, Matrimony Vine, Night Blooming Jessamine, Stinkweed.


| PHYSOSTIGMINE SALICYLATE
physostigmine salicylate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - General Injectables and Vaccines, Inc.a (108250663) |
