PRESTALIA- perindopril arginine, amlodipine besylate tablet
Marina BioTech
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRESTALIA® tablets safely and effectively. See full prescribing information for PRESTALIA.
PRESTALIA (perindopril arginine and amlodipine) tablets, for oral use. Initial U.S. Approval: 2015 INDICATIONS AND USAGEPrestalia is a combination of perindopril, an angiotensin converting enzyme inhibitor, and amlodipine, a dihydropyridine calcium channel blocker, indicated for the treatment of hypertension to lower blood pressure:
Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions (1). DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions were edema (7.2%), cough (3.2%), headache (2.5%), and dizziness (2.5%) (6.1). To report SUSPECTED ADVERSE REACTIONS, contact SYMPLMED LLC at 1-866-561-3088 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 3/2018 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
PRESTALIA contains perindopril arginine, an angiotensin converting enzyme inhibitor, and amlodipine, a dihydropyridine calcium channel blocker, and is indicated for the treatment of hypertension, to lower blood pressure.
PRESTALIA may be used in patients whose blood pressure is not adequately controlled on monotherapy.
PRESTALIA may be used as initial therapy in patients likely to need multiple drugs to achieve blood pressure goals.
Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions.
These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including amlodipine and the ACE inhibitor class to which perindopril principally belongs. There are no controlled trials demonstrating risk reduction with PRESTALIA.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. In a clinical trial of PRESTALIA, treatment with PRESTALIA 14/10 mg did not provide additional antihypertensive effect beyond that achieved with use of amlodipine 10 mg in black and diabetic patients [see Clinical Studies (14)].
The choice of PRESTALIA as initial therapy for hypertension should be based on an assessment of potential benefits and risks including whether the patient is likely to tolerate the starting dose of PRESTALIA.
Patients with moderate-to-severe hypertension are at a relatively high risk of cardiovascular events (e.g., stroke, heart attack, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. Consider the patient's baseline blood pressure, target goal and the incremental likelihood of achieving the goal with a combination product, such as PRESTALIA, versus a monotherapy product when deciding upon initial therapy. Individual blood pressure goals may vary based on the patient's risk.
Data from an 6-week, active-controlled trial provide estimates of the probability of reaching a target blood pressure with PRESTALIA compared with perindopril erbumine or amlodipine monotherapy [see Clinical Studies (14)].
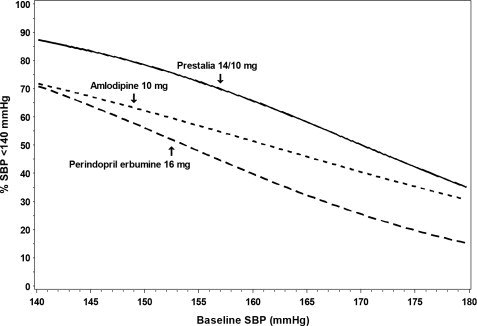
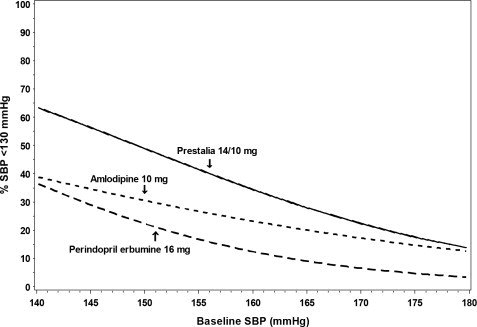
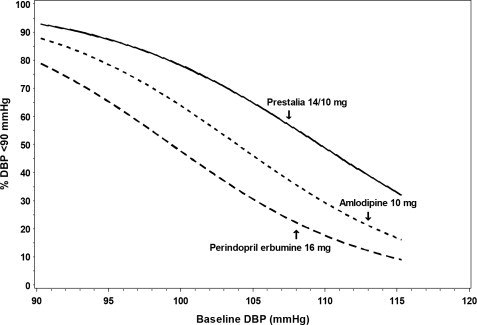
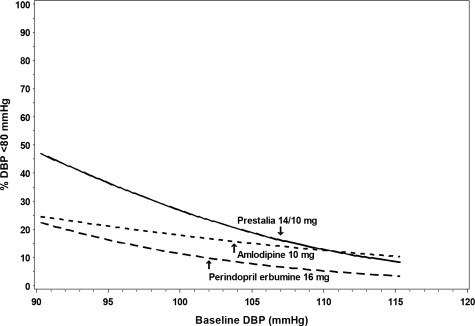
Figures 1.a-1.d provide estimates of the likelihood of achieving target clinic systolic and diastolic blood pressure control with PRESTALIA 14/10 mg tablets after 6 weeks, based on baseline systolic and diastolic blood pressure. The curve for each treatment group was estimated by logistic regression modeling and is less well defined in the tails.
Figure 1.a Probability of Achieving Systolic Blood Pressure <140 mmHg at Week 6
Figure 1.b Probability of Achieving Systolic Blood Pressure <130 mmHg at Week 6
Figure 1.c Probability of Achieving Diastolic Blood Pressure <90 mmHg at Week 6
Figure 1.d Probability of Achieving Diastolic Blood Pressure <80 mmHg at Week 6
For example, a patient with a baseline blood pressure of 170/105 mmHg has approximately a 26% likelihood of achieving a goal of <140 mmHg (systolic) and 31% likelihood of achieving <90 mmHg (diastolic) on perindopril erbumine 16 mg. The likelihood of achieving these same goals on amlodipine 10 mg is approximately 40% (systolic) and 46% (diastolic). These likelihoods rise to 50% (systolic) and 65% (diastolic) with PRESTALIA 14/10 mg.
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
The recommended starting dose of PRESTALIA is 3.5/2.5 mg once daily.
Adjust dosage according to blood pressure goals. In general, wait 7 to 14 days between titration steps. The maximum recommended dose is 14/10 mg once daily [see Clinical Pharmacology (12.3)].
PRESTALIA may be used as initial therapy if a patient is likely to need multiple drugs to achieve blood pressure goals.
Consider use in patients unable to achieve adequate antihypertensive effect with amlodipine monotherapy because of dose-limiting peripheral edema caused by amlodipine [see Adverse Reactions (6)].
Administered as monotherapy, perindopril erbumine is an effective treatment for hypertension in once-daily doses ranging from 4 mg to 16 mg daily. Amlodipine is effective in once-daily doses of 5 mg and 10 mg. Adverse reactions related to perindopril are generally uncommon and independent of dose, while those related to amlodipine are a mixture of dose-dependent phenomena (primarily peripheral edema) and dose-independent phenomena, the former much more common than the latter [see Adverse Reactions (6)].
2.2 Dosage Adjustment in Renal Impairment
PRESTALIA is not recommended in patients with creatinine clearances <30 mL/min. For patients with creatinine clearance between 30 and 80 mL/min (mild or moderate renal impairment), do not exceed 7/5 mg [see Use in Specific Populations (8.6) and Warnings and Precautions (5.7)].
3 DOSAGE FORMS AND STRENGTHS
PRESTALIA is available as fixed dose combination tablets of perindopril arginine and amlodipine:
- 3.5/2.5 mg tablets: white, uncoated tablets debossed with 3.5 on one side and 2.5 on the other side.
- 7/5 mg tablets: white, uncoated tablets debossed with 7/5 on one side and blank on the other side.
- 14/10 mg tablets: white, uncoated tablets debossed with 14/10 on one side and blank on the other side.
4 CONTRAINDICATIONS
PRESTALIA tablets are contraindicated in patients with hereditary or idiopathic angioedema, with or without previous ACE inhibitor treatment, and in patients who are hypersensitive to perindopril, to any other ACE inhibitor, or to amlodipine.
Do not co-administer aliskiren with ACE inhibitors, including PRESTALIA, in patients with diabetes.
Prestalia is contraindicated in combination with neprilysin inhibitor (e.g., sacubitril). Do not administer Prestalia within 36 hours of switching to or from sacubitril/valsartan, a neprilysin inhibitor [see Warnings and Precautions (5.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue PRESTALIA as soon as possible [see Use in Specific Populations (8.1)].
5.2 Anaphylactoid and Possibly Related Reactions
Angiotensin converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin. Patients taking ACE inhibitors (including the one in PRESTALIA) may, therefore, be subject to a variety of bradykinin- or prostaglandin-mediated adverse reactions, some of them serious. Black patients receiving ACE inhibitors have a higher incidence of angioedema compared with non-blacks.
Head and Neck Angioedema: Angioedema of the face, extremities, lips, tongue, glottis, and larynx has been reported in patients treated with ACE inhibitors (0.1% of patients treated with perindopril in U.S. clinical trials). Angioedema associated with involvement of the tongue, glottis or larynx may be fatal. In such cases, discontinue perindopril treatment immediately and observe until the swelling disappears. When involvement of the tongue, glottis, or larynx appears likely to cause airway obstruction, administer appropriate therapy, such as subcutaneous epinephrine solution 1:1000 (0.3 to 0.5 mL), promptly.
Patients taking concomitant mTOR inhibitor (e.g. temsirolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema [see Drug Interactions (7)].
Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting), and the angioedema was diagnosed by imaging studies such as abdominal CT or ultrasound, or at surgery. In some cases there was no prior history of facial angioedema, and C-1 esterase levels were normal. Symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
5.3 Increased Angina and/or Myocardial Infarction
Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of PRESTALIA, particularly in patients with severe obstructive coronary artery disease.
5.4 Hypotension
PRESTALIA can cause symptomatic hypotension. Symptomatic hypotension is most likely to occur in patients who have been volume- or salt-depleted as a result of prolonged diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting.
In patients at risk of excessive hypotension, start PRESTALIA therapy under close medical supervision. Follow patients closely for the first 2 weeks of treatment and whenever the dose of PRESTALIA is increased or a diuretic is added or its dose increased.
If excessive hypotension occurs, immediately place patient in a supine position and, if necessary, treat patient with an intravenous infusion of physiological saline. PRESTALIA treatment can usually be continued following restoration of volume and blood pressure.
Patients with severe aortic stenosis may be more likely to experience symptomatic hypotension. Because of the gradual onset of action, acute hypotension is unlikely.
Surgery/Anesthesia
In patients undergoing major surgery or during anesthesia with agents that produce hypotension, PRESTALIA may block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
5.5 Hyperkalemia
Elevations of serum potassium have been observed in some patients treated with ACE inhibitors, including PRESTALIA. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of agents such as potassium-sparing diuretics, potassium supplements, and/or potassium-containing salt substitutes [see Drug Interactions (7)].
Monitor serum potassium periodically in patients receiving PRESTALIA.
5.6 Cough
Presumably because of the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, generally resolving after discontinuation of therapy. Consider ACE inhibitor-induced cough in the differential diagnosis of cough.
5.7 Impaired Renal Function
Monitor renal function periodically in patients receiving PRESTALIA. Drugs that affect the renin-angiotensin system can cause reductions in renal function, including acute renal failure. Patients whose renal function may depend in part on the activity of the renin-angiotensin system—(e.g., patients with renal artery stenosis, severe heart failure, post-myocardial infarction or volume depletion) or who are on non-steroidal anti-inflammatory agents (NSAIDS) or angiotensin receptor blockers—may be at particular risk of developing acute renal failure on PRESTALIA. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on PRESTALIA.
5.8 Hepatic Failure
Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and sometimes death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In an active-controlled 6-week trial, the safety of the maximum dose of PRESTALIA (14/10 mg) was evaluated in 279 patients with hypertension and compared with perindopril erbumine 16 mg and amlodipine 10 mg. Adverse reactions were generally mild and transient in nature.
Discontinuations because of adverse events occurred in 3.6% of patients treated with PRESTALIA 14/10 mg compared to 4.3% of patients treated with perindopril erbumine 16 mg and 4.6% of patients treated with amlodipine 10 mg. The most common reason for discontinuation of therapy with PRESTALIA was peripheral edema (1.8%).
Common adverse events that occurred in at least 2% of patients treated with PRESTALIA in the 6-week trial are presented in Table 1.
|
PERe = perindopril erbumine; AML = amlodipine besylate |
|||
| Adverse Event | PRESTALIA 14/10 mg
(N = 279) n (%) | PERe 16 mg
(N = 278) n (%) | AML 10 mg
(N = 280) n (%) |
| Edema peripheral | 20 (7.2) | 1 (0.4) | 37 (13.2) |
| Cough | 9 (3.2) | 8 (2.9) | 2 (0.7) |
| Headache | 7 (2.5) | 8 (2.9) | 8 (2.9) |
| Dizziness | 7 (2.5) | 4 (1.4) | 3 (1.1) |
The overall frequency of adverse reactions was similar between men and women, and black and non-black patients. In black patients, the incidence of peripheral edema was similar in the PRESTALIA 14/10 mg and amlodipine 10 mg arms (3%).
Other adverse reactions in the controlled clinical trial with some plausible relationship to PRESTALIA are listed below.
Dermatologic: Rash
Digestive: Nausea, diarrhea
The safety of the lowest dose of PRESTALIA (3.5/2.5 mg) was evaluated in 249 patients with hypertension and compared with placebo and perindopril and amlodipine administered as monotherapies in an 8-week trial. The only emergent adverse event observed in at least 2% of patients treated with PRESTALIA was hyperkalemia (2.4%). Peripheral edema was reported in 1.6% of patients receiving PRESTALIA 3.5/2.5 mg.
Monotherapy with perindopril or amlodipine has been evaluated for safety in clinical trials in over 3,000 and 11,000 patients, respectively, as summarized below.
Perindopril
Perindopril erbumine has been evaluated for safety in approximately 3,400 patients with hypertension in U.S. and foreign clinical trials. The data presented here are based on results from the 1,417 perindopril-treated patients who participated in the U.S. clinical trials. Over 220 of these patients were treated with perindopril for at least one year.
In placebo-controlled U.S. clinical trials, the incidence of premature discontinuation of therapy due to adverse events was 6.5% in patients treated with perindopril erbumine and 6.7% in patients treated with placebo. The most common causes were cough, headache, asthenia, and dizziness.
Among 1,012 patients in placebo-controlled U.S. trials, the overall frequency of reported adverse events was similar in patients treated with perindopril erbumine and in those treated with placebo (approximately 75% in each group). The only adverse events whose incidence on perindopril erbumine was at least 2% greater than on placebo were cough (12% vs. 4.5%) and back pain (5.8% vs. 3.1%).
Dizziness was not reported more frequently in the perindopril group (8.2%) than in the placebo group (8.5%), but its likelihood increased with dose, suggesting a causal relationship with perindopril.
Amlodipine
Amlodipine has been evaluated for safety in more than 11,000 patients in U.S. and foreign clinical trials. In controlled clinical trials comparing amlodipine (N=1730) in doses up to 10 mg with placebo (N=1250), discontinuation of amlodipine due to adverse reactions was required in about 1.5% of amlodipine-treated patients and about 1% of placebo-treated patients. The most common side effects were edema, dizziness, flushing, and palpitations.
The following events occurred in <1% but >0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the physician to a possible relationship:
Cardiovascular: arrhythmia (including ventricular tachycardia and atrial fibrillation), bradycardia, chest pain, peripheral ischemia, syncope, tachycardia, vasculitis.
Central and Peripheral Nervous System: hypoesthesia, neuropathy peripheral, paresthesia, tremor, vertigo.
Gastrointestinal: anorexia, constipation, dysphagia, diarrhea, flatulence, pancreatitis, vomiting, gingival hyperplasia.
General: allergic reaction, asthenia,1 back pain, hot flushes, malaise, pain, rigors, weight gain, weight decrease.
Musculoskeletal System: arthralgia, arthrosis, muscle cramps,1 myalgia.
Psychiatric: sexual dysfunction (male1 and female), insomnia, nervousness, depression, abnormal dreams, anxiety, depersonalization.
Respiratory System: dyspnea,1 epistaxis.
Skin and Appendages: angioedema, erythema multiforme, pruritus,1 rash,1 rash erythematous, rash maculopapular.
Special Senses: abnormal vision, conjunctivitis, diplopia, eye pain, tinnitus.
Urinary System: micturition frequency, micturition disorder, nocturia.
Autonomic Nervous System: dry mouth, sweating increased.
Metabolic and Nutritional: hyperglycemia, thirst.
Hematopoietic: leukopenia, purpura, thrombocytopenia.
1 These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
Clinical Laboratory Findings
Perindopril
Hematology: Small decreases in hemoglobin and hematocrit occur frequently in hypertensive patients treated with perindopril, but are rarely of clinical importance. In controlled clinical trials, no patient was discontinued from therapy due to the development of anemia. Leukopenia (including neutropenia) was observed in 0.1% of patients in U.S. clinical trials.
Liver Function Tests: Elevations in alanine transaminase (ALT; 1.6% perindopril erbumine vs. 0.9% placebo) and aspartate transaminase (AST; 0.5% perindopril erbumine vs. 0.4% placebo) have been observed in placebo-controlled clinical trials. The elevations were generally mild and transient and resolved after discontinuation of therapy.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of the individual components of PRESTALIA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Perindopril: Voluntary reports of adverse events in patients taking perindopril that have been received since market introduction and are of unknown causal relationship to perindopril include: cardiac arrest, eosinophilic pneumonitis, neutropenia/agranulocytosis, pancytopenia, anemia (including hemolytic and aplastic), thrombocytopenia, acute renal failure, nephritis, hepatic failure, jaundice (hepatocellular or cholestatic), symptomatic hyponatremia, bullous pemphigoid, pemphigus, acute pancreatitis, falls, psoriasis, exfoliative dermatitis, and a syndrome that may include: arthralgia/arthritis, vasculitis, serositis, myalgia, fever, rash or other dermatologic manifestations, a positive antinuclear antibody (ANA), leukocytosis, eosinophilia, or an elevated erythrocyte sedimentation rate (ESR).
7 DRUG INTERACTIONS
Prestalia
The pharmacokinetics of perindopril and amlodipine are not altered when the drugs are co-administered.
No drug interaction studies have been conducted with PRESTALIA, although studies have been conducted with perindopril and amlodipine.
mTOR Inhibitors: Patients taking concomitant mTOR inhibitor (e.g. temsirolimus) therapy may be at increased risk for angioedema. [see Warnings and Precautions (5.2)]
Neprilysin Inhibitor: Patients taking concomitant neprilysin inhibitors may be at increased risk for angioedema. [see Warnings and Precautions (5.2)]
Perindopril
Diuretics:Patients on diuretics, especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with PRESTALIA. Provide close medical supervision with the first dose of PRESTALIA, for at least two hours and until blood pressure has stabilized for another hour. Perindopril can attenuate potassium loss caused by thiazide diuretics.
Potassium Supplements and Potassium-Sparing Diuretics:Potassium-sparing diuretics (spironolactone, amiloride, triamterene, and others) or potassium supplements, or other drugs capable of increasing serum potassium (indomethacin, heparin, cyclosporine and others) can increase the risk of hyperkalemia. If concomitant use of such agents is indicated, the patient's serum potassium should be monitored frequently.
Lithium:Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving ACE inhibitors during therapy with lithium. When co-administering PRESTALIA and lithium, frequent monitoring of serum lithium levels is recommended. Use of a diuretic may further increase the risk of lithium toxicity.
Gold:Nitritoid reactions (symptoms include facial flushing, nausea, vomiting, and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy.
Non-Steroidal Anti-Inflammatory Agents (NSAIDS) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors):In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDS, including selective COX-2 inhibitors, with ACE inhibitors, including perindopril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving perindopril and NSAID therapy.
The antihypertensive effects of ACE inhibitors, including perindopril, may be attenuated by NSAIDS, including selective COX-2 inhibitors.
Dual Blockade of the Renin-Angiotensin System (RAS):Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. In most patients no benefit has been associated with using two RAS inhibitors concomitantly. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function, and electrolytes in patients on PRESTALIA and other agents that affect the RAS.
Do not co-administer aliskiren with PRESTALIA in patients with diabetes. Avoid use of aliskiren with PRESTALIA in patients with renal impairment (GFR <60 mL/min).
Amlodipine
Simvastatin: Co-administration of multiple doses of 10 mg of amlodipine with 80 mg simvastatin resulted in a 77% increase in exposure to simvastatin compared to simvastatin administered alone. Limit the dose of simvastatin in patients on amlodipine to 20 mg daily.
Cyclosporine: A prospective study in renal transplant patients showed an average 40% increase in trough cyclosporin levels during concomitant treatment with amlodipine. Frequent monitoring of trough blood levels of cyclosporine is recommended.
CYP3A Inhibitors: Co-administration of the moderate CYP3A inhibitor diltiazem increases the exposure to amlodipine by 60%. Co-administered erythromycin, also a moderate CYP3A inhibitor, does not impact the exposure to amlodipine. Strong CYP3A inhibitors (e.g., itraconazole) may increase the plasma concentrations of the CYP3A substrate amlodipine to a greater extent. Monitor for symptoms of hypotension and edema when amlodipine is co-administered with moderate or strong CYP3A inhibitors to determine the need for dose adjustment.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D [see Warnings and Precautions (5.1)]
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue PRESTALIA as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultra-sound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue PRESTALIA, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to PRESTALIA for hypotension, oliguria, and hyperkalemia [see Use in Specific Populations (8.4)].
Radioactivity was detectable in fetuses after administration of 14C-perindopril to pregnant rats.
8.3 Nursing Mothers
It is not known whether perindopril or amlodipine is excreted in human milk, but radioactivity was detected in the milk of lactating rats following administration of 14C-perindopril. Because of the potential for adverse effects on the nursing infant, decide whether to discontinue nursing or discontinue PRESTALIA.
8.4 Pediatric Use
Neonates with a history of in utero exposure to PRESTALIA:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
The safety and effectiveness of PRESTALIA in pediatric patients have not been established.
8.5 Geriatric Use
The mean blood pressure effect of perindopril was somewhat smaller in patients over 60 years of age than in younger patients, although the difference was not significant. Plasma concentrations of both perindopril and perindoprilat in elderly patients (>65 years) are approximately twice those observed in younger patients. No adverse effects were clearly increased in older patients with the exception of dizziness and rash.
Amlodipine is extensively metabolized in the liver. In the elderly, clearance of amlodipine is decreased with resulting increases in peak plasma levels, elimination half-life, and AUC.
Experience with PRESTALIA is limited in the elderly at doses exceeding 7/5 mg. If doses above 7/5 mg are required, monitor blood pressure up to two weeks following up titration [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Pharmacokinetic data indicate that perindoprilat elimination is decreased in renally impaired patients, with a marked increase in accumulation when creatinine clearance drops below 30 mL/min. PRESTALIA is not recommended in patients with creatinine clearances <30 mL/min. For patients with creatinine clearance between 30 and 80 mL/min (mild or moderate renal impairment), do not exceed 7/5 mg [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Perindopril
In animals, doses of perindopril up to 2,500 mg/kg in mice, 3,000 mg/kg in rats and 1,600 mg/kg in dogs were non-lethal. Past experiences were scant but suggested that overdosage with other ACE inhibitors was also fairly well tolerated by humans. The most likely manifestation is hypotension, and treatment should be symptomatic and supportive. Therapy with the ACE inhibitor should be discontinued, and the patient should be observed. Dehydration, electrolyte imbalance and hypotension should be treated by established procedures.
Among the reported cases of perindopril overdosage, patients who were known to have ingested a dose of 80 mg to 120 mg required assisted ventilation and circulatory support. One additional patient developed hypothermia, circulatory arrest and died following ingestion of up to 180 mg of perindopril. The intervention for perindopril overdose may require vigorous support.
Laboratory determinations of serum levels of perindopril and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of perindopril overdose. No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of perindopril and its metabolites.
Angiotensin II could presumably serve as a specific antagonist-antidote in the settling of perindopril overdose, but angiotensin II is essentially unavailable outside of scattered research facilities. Because the hypotensive effect of perindopril is achieved through vasodilation and effective hypovolemia, it is reasonable to treat perindopril overdose by infusion of normal saline solution.
Amlodipine
Overdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. In humans, experience with intentional overdosage of amlodipine is limited.
Single oral doses of amlodipine maleate equivalent to 40 mg amlodipine/kg and 100 mg amlodipine/kg in mice and rats, respectively, caused deaths. Single oral amlodipine maleate doses equivalent to 4 or more mg amlodipine/kg or higher in dogs (11 or more times the maximum recommended human dose on a mg/m2 basis) caused a marked peripheral vasodilation and hypotension.
If massive overdose should occur, initiate active cardiac and respiratory monitoring. Frequent blood pressure measurements are essential. Should hypotension occur, provide cardiovascular support including elevation of the extremities and the judicious administration of fluids. If hypotension remains unresponsive to these conservative measures, consider administration of vasopressors (such as phenylephrine) with attention to circulating volume and urine output. As amlodipine is highly protein bound, hemodialysis is not likely to be of benefit.
11 DESCRIPTION
PRESTALIA is a combination of perindopril arginine and amlodipine besylate.
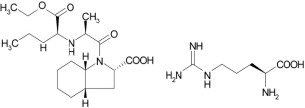
Perindopril arginine is the L-arginine salt of perindopril, the ethyl ester of a non-sulfhydryl angiotensin converting enzyme inhibitor. Perindopril arginine is chemically described as L-arginine (2S,3aS,7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl)butyl]amino]propanoyl] octahydro-1H-indole-2-carboxylate. Its empirical formula is C19H32N2O5•C6H14N4O2 and its structural formula is:
Perindopril arginine is a white, crystalline powder with a molecular weight 542.7. The free acid has the molecular weight of 368.5. It is readily soluble in purified water, slightly soluble in 95% ethanol, and practically insoluble in chloroform.
Perindopril is the free-acid form of perindopril arginine. Perindopril is a pro-drug and is metabolized in vivo by hydrolysis of the ester group to form perindoprilat, the biologically active metabolite.
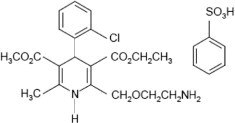
Amlodipine besylate is the benzene sulphonic acid salt of amlodipine, a long-acting dihydropyridine calcium channel blocker. Amlodipine besylate is chemically described as 3-ethyl-5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate monobenzenesulphonate. Its empirical formula is C20H25ClN2O5•C6H6O3S and its structural formula is:
Amlodipine besylate is a white crystalline powder with a molecular weight of 567.1. It is slightly soluble in water and sparingly soluble in ethanol. The content of the tablets is expressed as amlodipine (free base) which has a molecular weight of 409.1.
PRESTALIA tablets are formulated in three different strengths for oral administration. Tablets contain perindopril arginine 3.5 mg, 7 mg, or 14 mg and amlodipine 2.5 mg, 5 mg, or 10 mg for the following available perindopril arginine/amlodipine combinations: 3.5/2.5 mg, 7/5 mg, and 14/10 mg.
Inactive ingredients are lactose, microcrystalline cellulose, colloidal silicon dioxide, and magnesium stearate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Perindopril
Perindopril, a pro-drug, is hydrolyzed to perindoprilat, which inhibits ACE in humans and in animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of the inactive decapeptide, angiotensin I, to the vasoconstrictor substance angiotensin II. Angiotensin II is a potent peripheral vasoconstrictor, which stimulates aldosterone secretion by the adrenal cortex, and provides negative feedback on renin secretion. Inhibition of ACE results in decreased plasma angiotensin II, leading to decreased vasoconstriction, increased plasma renin activity and decreased aldosterone secretion. The latter results in diuresis and natriuresis and may be associated with an increase in serum potassium [see Warnings and Precautions (5.6)].
ACE is identical to kininase II, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of perindopril remains to be elucidated.
While the principal mechanism of perindopril in blood pressure reduction is believed to be through the renin-angiotensin-aldosterone system, ACE inhibitors have some effect even in apparent low-renin hypertension. Perindopril has been studied in relatively few black patients, usually a low-renin population, and the average response of diastolic blood pressure to perindopril was about half the response seen in nonblack patients, a finding consistent with previous experience of other ACE inhibitors.
Amlodipine
Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow channel blocker) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected in vitro but such effects have not been seen in intact animals at therapeutic doses.
Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect.
Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure.
12.2 Pharmacodynamics
Perindopril
After administration of perindopril, ACE is inhibited in a dose and blood concentration-related fashion. The degree of ACE inhibition achieved by a given dose appears to diminish over time (the ID50 increases). The pressor response to an angiotensin I infusion is reduced by perindopril, but this is not as persistent as the effect on ACE.
Amlodipine
Following administration of therapeutic doses to patients with hypertension, amlodipine produces vasodilation resulting in a reduction of supine and standing blood pressures. These decreases in blood pressure are not accompanied by a significant change in heart rate or plasma catecholamine levels with chronic dosing. Although the acute intravenous administration of amlodipine decreases arterial blood pressure and increases heart rate in hemodynamic studies of patients with chronic stable angina, chronic oral administration of amlodipine in clinical trials did not lead to clinically significant changes in heart rate or blood pressures in normotensive patients with angina.
With chronic once daily oral administration, antihypertensive effectiveness is maintained for at least 24 hours. Plasma concentrations correlate with effect in both young and elderly patients. The magnitude of reduction in blood pressure with amlodipine is also correlated with the height of pretreatment elevation; thus, individuals with moderate hypertension (diastolic pressure 105-114 mmHg) had about a 50% greater response than did patients with mild hypertension (diastolic pressure 90-104 mmHg). Normotensive subjects experienced no clinically significant change in blood pressures (+1/-2 mmHg).
In hypertensive patients with normal renal function, therapeutic doses of amlodipine resulted in a decrease in renal vascular resistance and an increase in glomerular filtration rate and effective renal plasma flow without change in filtration fraction or proteinuria.
As with other calcium channel blockers, hemodynamic measurements of cardiac function at rest and during exercise (or pacing) in patients with normal ventricular function treated with amlodipine have generally demonstrated a small increase in cardiac index without significant influence on dP/dt or on left ventricular end diastolic pressure or volume. In hemodynamic studies, amlodipine has not been associated with a negative inotropic effect when administered in the therapeutic dose range to intact animals and humans, even when co-administered with β-blockers to humans. Similar findings, however, have been observed in normal or well-compensated patients with heart failure with agents possessing significant negative inotropic effects.
Electrophysiologic Effects: Amlodipine does not change sinoatrial (SA) nodal function or atrioventricular (AV) conduction in intact animals or humans. In clinical studies in which amlodipine was administered in combination with β-blockers to patients with either hypertension or angina, no adverse effects on electrocardiographic parameters were observed.
12.3 Pharmacokinetics
Prestalia
Following administration of PRESTALIA, peak plasma concentration of perindopril, perindoprilat and amlodipine occur at approximately 1 hour, 4 hours and 6-12 hours, respectively. The mean half-life of perindopril is approximately 1.3 hours. The decline in the plasma concentration of perindoprilat is multiphasic and shows a terminal elimination half-life of approximately 100 hours, resulting from slow dissociation of perindoprilat from plasma/tissue ACE binding sites. Amlodipine elimination from plasma is biphasic with a terminal elimination half-life of approximately 30 to 50 hours.
When PRESTALIA is administered with food, the exposure to perindopril, perindoprilat and amlodipine is not impacted.
Perindopril
Following administration of PRESTALIA, perindopril is rapidly absorbed, with peak plasma concentrations occurring at approximately 1 hour. The absolute oral bioavailability of perindopril is approximately 75%. Following absorption, approximately 30% to 50% of systemically available perindopril is hydrolyzed to its active metabolite, perindoprilat, which has a mean bioavailability of approximately 25%. Peak plasma concentrations of perindoprilat are attained approximately 4 hours after PRESTALIA administration. Food had no effect on the extent of absorption of perindopril or perindoprilat, but slightly reduced the rate of absorption of perindopril and perindoprilat by 18% and 14%, respectively.
The Cmax and AUC of perindopril and perindoprilat increase in a linear and dose proportional manner following both single oral dosing and at steady state during an once-a-day multiple dosing regimen. Perindopril exhibits multiexponential pharmacokinetics following oral administration. The mean half-life of perindopril associated with most of its elimination is approximately 0.8 to 1 hours.
Perindopril is extensively metabolized following oral administration, with only 4% to 12% of the dose recovered unchanged in the urine. Six metabolites resulting from hydrolysis, glucuronidation, and cyclization via dehydration have been identified. These include the active ACE inhibitor perindoprilat (hydrolyzed perindopril), perindopril, and perindoprilat glucuronides, dehydrated perindopril, and the diastereoisomers of dehydrated perindoprilat. In humans, hepatic esterase appears to be responsible for the hydrolysis of perindopril.
The active metabolite, perindoprilat, also exhibits multiexponential pharmacokinetics following the oral administration of perindopril. Formation of perindoprilat is gradual with peak plasma concentrations occurring between 3 and 7 hours. The subsequent decline in plasma concentration shows a prolonged terminal elimination half-life of 120 hours resulting from slow dissociation of perindoprilat from plasma/tissue ACE binding sites. During repeated oral once-daily dosing with perindopril, perindoprilat accumulates about 1.5- to 2-fold and attains steady state plasma levels in 3 to 6 days. The clearance of perindoprilat and its metabolites is almost exclusively renal.
Approximately 60% of circulating perindopril is bound to plasma proteins, and only 10% to 20% of perindoprilat is bound. Therefore, drug interactions mediated through effects on protein binding are not anticipated.
Amlodipine
Absolute bioavailability of amlodipine has been estimated between 64% and 90%. Ex vivo studies indicate that approximately 93% of circulating amlodipine is bound to plasma proteins in hypertensive patients.
Amlodipine is extensively (approximately 90%) metabolized in the liver to inactive metabolites. Steady-state plasma levels are reached after once-daily dosing for 7 to 8 days. 10% of unchanged drug and 60% of amlodipine metabolites are excreted in urine.
Drug Interactions:
Perindopril: Co-administered perindopril does not impact the exposure to amlodipine or digoxin.
Amlodipine: Co-administered cimetidine, magnesium- and aluminum hydroxide antacids, sildenalfil, and grapefruit juice have no impact on the exposure to amlodipine. Co-administered amlodipine does not affect the exposure to perindopril, perindoprilat, atorvastatin, ethanol and the warfarin prothrombin response time.
Specific Populations
Elderly:
Perindopril: Plasma concentrations of both perindopril and perindoprilat in elderly patients (>65 years) are approximately twice those observed in younger patients, reflecting both increased conversion of perindopril to perindoprilat and decreased renal excretion of perindoprilat [see Dosing and Administration (2.2)and Use in Specific Populations (8.5)].
Renal Impairment:
Perindopril: Perindoprilat elimination is decreased in renally impaired patients. At creatinine clearances of 30 mL/min to 80 mL/min, AUC is about double that at 100 mL/min. When creatinine clearance drops below 30 mL/min, AUC increases more markedly [see Dosing and Administration (2.2) and Warnings and Precautions (5.7)].
During dialysis, perindopril is removed with the same clearance as in patients with normal renal function. In a limited number of patients studied, perindopril clearance by dialysis ranged from about 40 to 80 mL/min. Perindoprilat clearance by dialysis ranged from about 40 to 90 mL/min [see Dosing and Administration (2.2)].
Hepatic Impairment:
Perindopril: The bioavailability of perindoprilat is increased in patients with impaired hepatic function. Plasma concentrations of perindoprilat in patients with impaired liver function were about 50% higher than those observed in healthy subjects or hypertensive patients with normal liver function [see Warnings and Precautions (5.8)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity, mutagenicity or fertility studies have been conducted with the combination of perindopril and amlodipine. However, these studies have been conducted for perindopril and amlodipine alone.
Perindopril
Carcinogenicity: No evidence of carcinogenicity was observed in studies in rats and mice when perindopril was administered at dosages up to 5 times (mg/m2) the maximum recommended human dose (MRHD) of 14 mg/day for 104 weeks.
Mutagenesis: No genotoxic potential was detected for perindopril, perindoprilat, and other metabolites in various in vitro and in vivo investigations, including the Ames test, the Saccharomyces cerevisiae D4 test, cultured human lymphocytes, thymidine kinase ± mouse lymphoma assay, mouse and rat micronucleus tests, the in vivo micronucleus and chromosomal aberration tests, and Chinese hamster bone marrow assay.
Amlodipine
Carcinogenicity: Rats and mice treated with amlodipine maleate in the diet for up to 2 years, at concentrations calculated to provide daily amlodipine dosage levels of 0.5, 1.25, and 2.5 mg/kg/day, showed no evidence of a carcinogenic effect of the drug. For the mouse, the highest dose was, on a body surface area basis, similar to the amlodipine MRHD of 10 mg/day. For the rat, the highest dose was, on a body surface area basis, approximately 2.5 times the MRHD, assuming a patient weight of 60 kg.
13.3 Reproductive Toxicity
Reproductive toxicity studies have not been conducted with this combination. However, these studies have been conducted for amlodipine alone.
Amlodipine
No evidence of teratogenicity or other embryo/fetal toxicity was found when pregnant rats and rabbits were treated orally with amlodipine maleate at amlodipine doses of up to 10 mg/kg/day (respectively, about 8 and 23 times the maximum recommended human dose of 10 mg on a mg/m2 basis, assuming a patient weight of 50 kg) during their periods of major organogenesis. However, litter size was significantly decreased (by about 50%) and the number of intrauterine deaths was significantly increased (about 5-fold) for rats receiving amlodipine maleate at an amlodipine dose equivalent to 10 mg/kg/day for 14 days before mating and throughout mating and gestation. Amlodipine maleate has been shown to prolong both the gestation period and the duration of labor in rats at this dose.
14 CLINICAL STUDIES
The antihypertensive effects of PRESTALIA have been demonstrated in two randomized controlled trials.
The highest strength of PRESTALIA (14/10 mg) was studied in a double-blind, active controlled study in hypertensive patients. A total of 837 patients with seated diastolic pressure 95 to 115 mmHg (mean baseline systolic/diastolic blood pressure was 158/101 mmHg) received treatments of PRESTALIA 14/10 mg, perindopril erbumine 16 mg, or amlodipine 10 mg once daily for 6 weeks. The mean age of the population was 51 years, 51% of patients were male, and 34% were black. Overall, 20% of the population had type 2 diabetes.
At Week 6, PRESTALIA 14/10 mg produced statistically significantly greater reductions in blood pressure than each of the monotherapies. The reductions in systolic/diastolic blood pressure with PRESTALIA 14/10 mg were 10.1/6.3 mmHg greater than with perindopril erbumine 16 mg and 3.9/2.5 mmHg greater than with amlodipine 10 mg. In black patients and in diabetic patients, treatment with PRESTALIA 14/10 mg did not provide additional antihypertensive effect beyond that achieved with use of amlodipine 10 mg.
The lowest strength of perindopril arginine/amlodipine (3.5/2.5 mg) was studied in 246 hypertensive patients. A total of 1581 patients with supine diastolic pressure 95-110 mmHg (mean baseline systolic/diastolic blood pressure was 161/101 mmHg) received treatment with perindopril arginine/amlodipine 3.5/2.5 mg, perindopril arginine 3.5 mg, perindopril arginine 5 mg, amlodipine 2.5 mg, amlodipine 5 mg, or placebo. The mean age of the population was 52 years, 47% were male, and 1% were black. No included patients had a history of diabetes.
At Week 8, PRESTALIA 3.5/2.5 mg produced statistically significantly greater reductions in blood pressure than perindopril arginine 3.5 mg and amlodipine 2.5 mg. The reduction in systolic/diastolic blood pressure with perindopril arginine/amlodipine 3.5/2.5 mg was 7.2/4.1 mmHg greater than with placebo.
16 HOW SUPPLIED/STORAGE AND HANDLING
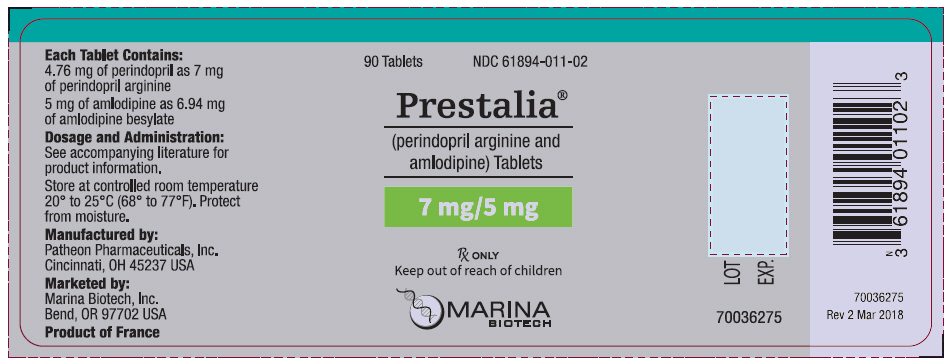
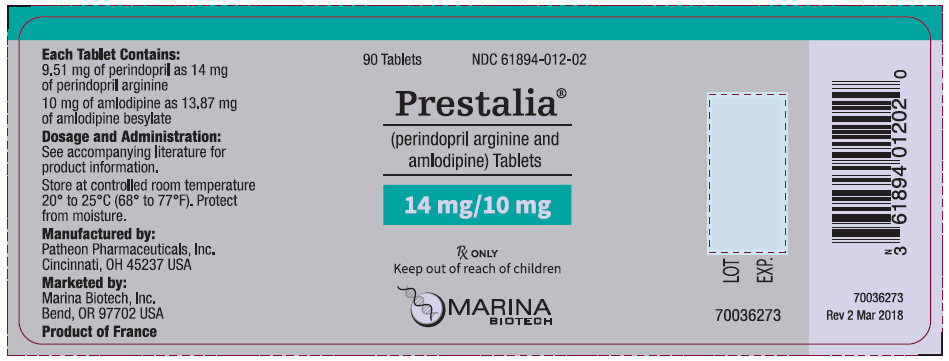
PRESTALIA is available as white, uncoated tablets containing perindopril arginine 3.5 mg, 7 mg, or 14 mg and amlodipine 2.5 mg, 5 mg, or 10 mg for the following three combinations of perindopril arginine/amlodipine: 3.5/2.5 mg, 7/5 mg, and 14/10 mg. All three strengths are packaged in bottles of 90 tablets. Each tablet is debossed with the tablet strength.
| Dose
(perindopril arginine, mg/amlodipine, mg) | Imprint | NDC Code |
| 3.5/2.5 | 3.5 imprinted on one side, 2.5 imprinted on the other side | NDC 61894-010-02 |
| 7/5 | 7/5 on one side, blank on the other | NDC 61894-011-02 |
| 14/10 | 14/10 on one side, blank on the other | NDC 61894-012-02 |
Storage: Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). [See USP controlled room temperature.] Protect from moisture. Dispense in tight container (USP).
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Tell female patients of childbearing age that use of drugs like perindopril that act on the renin-angiotensin system can cause serious problems in the fetus and infant, including low blood pressure, poor development of skull bones, kidney failure, and death. Discuss other treatment options with female patients planning to become pregnant. Tell women using PRESTALIA who become pregnant to notify their physician as soon as possible.
In case of a missed dose, have patients resume the usual dose at the next scheduled time.
Patient Information
PRESTALIA®(pres-ta-li-a)
(perindopril arginine and amlodipine)
tablets
Read this Patient Information before you start taking PRESTALIA and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is the most important information I should know about PRESTALIA?
- PRESTALIA can cause harm or death to your unborn baby.
- Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant.
- If you become pregnant while taking PRESTALIA, tell your doctor right away. Your doctor may switch you to a different medicine to treat your high blood pressure.
What is PRESTALIA?
PRESTALIA is a prescription medicine that contains perindopril arginine, an angiotensin converting enzyme inhibitor (ACE inhibitor), and amlodipine, a calcium channel blocker.
PRESTALIA is used to treat high blood pressure (hypertension):
- when one medicine to lower your high blood pressure is not enough
- as the first medicine to lower your high blood pressure if your doctor decides you are likely to need more than one medicine
It is not known if PRESTALIA is safe and effective in children.
Who should not take PRESTALIA?
Do not take PRESTALIA if you:
- have a history of angioedema. Symptoms of angioedema may include swelling of your face, tongue or throat, trouble breathing, itching, hives or skin rash, and stomach (abdominal) pain.
- are allergic to perindopril, or any other ACE inhibitor medicine
- are allergic to amlodipine
- have diabetes and take a medicine that contains aliskiren
What should I tell my doctor before taking PRESTALIA?
Before you take PRESTALIA, tell your doctor about all of your medical conditions, including if you:
- have heart, liver or kidney problems
- have diabetes
- have been told that you have abnormal potassium levels in your blood
- are vomiting or have diarrhea
- plan to have a surgical procedure
- are pregnant or plan to become pregnant. See "What is the most important information I should know about PRESTALIA?"
- are breastfeeding or plan to breastfeed. It is not known if PRESTALIA passes into your breast milk. You and your doctor should decide if you will take PRESTALIA or breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking PRESTALIA with other medicines can cause serious side effects.
Especially tell your doctor if you take:
- medicines for high blood pressure or heart problems
- water pills
- salt substitute
- potassium-containing medicines, potassium supplements, or salt substitutes containing potassium
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take PRESTALIA?
- Take PRESTALIA exactly as your doctor tells you.
- Take PRESTALIA 1 time each day, with or without food.
- If you miss a dose, take it as soon as you remember. If it is more than 12 hours, just take your next dose at the regular time.
- If you take too much PRESTALIA, call your doctor or go to the nearest emergency room right away.
What are the possible side effects of PRESTALIA?
PRESTALIA can cause serious side effects, including:
See "What is the most important information I should know about PRESTALIA?"
-
Serious allergic reactions that can be life threatening. Stop taking PRESTALIA and get emergency medical help right away if you get any of these symptoms of a serious allergic reaction:
- swelling of your face, lips, tongue, throat, arms, hands, legs, or feet
- trouble swallowing
- trouble breathing
- stomach (abdomen) pain with or without nausea or vomiting
People who are black and take PRESTALIA have a greater risk of having a serious allergic reaction than people who are not black and take PRESTALIA. - Worsening of chest pain (angina) or a heart attack (myocardial infarction) can happen after you start taking or increase your dose of PRESTALIA. Get emergency help if you get worse chest pain or chest pain that does not go away.
-
Low blood pressure (hypotension) is most likely to happen if you also:
- take water pills (diuretics)
- are on a low salt diet
- are on kidney dialysis
- have heart problems
- have vomiting or diarrhea
If you feel faint or dizzy, lie down and call your doctor right away. - Increased amount of potassium in the blood. Your doctor will check your potassium blood level during your treatment with PRESTALIA.
- Cough.
- Kidney problems. Some people with certain conditions may develop kidney problems and may need to stop treatment with PRESTALIA. Call your doctor if you get swelling in your feet, ankles, or hands, or unexplained weight gain.
The most common side effects of PRESTALIA include:
- swelling of the feet, ankles, and hands
- cough
- headache
- dizziness
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of PRESTALIA. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store PRESTALIA?
- Store PRESTALIA at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep PRESTALIA in a tightly closed container and in a dry place.
Keep PRESTALIA and all medicines out of the reach of children.
General information about PRESTALIA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use PRESTALIA for a condition for which it was not prescribed. Do not give PRESTALIA to other people, even if they have the same symptoms that you have. It may harm them.
For more information, go to www.prestalia-us.com or call 1-866-561-3088.
What is high blood pressure (hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too great.
High blood pressure makes the heart work harder to pump blood through the body and causes damage to the blood vessels. PRESTALIA can help your blood vessels relax so your blood pressure is lower. Medicines that lower your blood pressure lower your chance of having a stroke or heart attack.
What are the ingredients in PRESTALIA?
Active ingredients: perindopril arginine and amlodipine besylate
Inactive ingredients: lactose, microcrystalline cellulose, colloidal silicon dioxide, and magnesium stearate
This Patient Information has been approved by the U.S. Food and Drug Administration.
Marketed by:
Marina Biotech, Inc.
Bend, OR 97702
Rev: 03/2018
PRESTALIA® is a registered trademark of Biofarma used by Symplmed Pharmaceutics, LLC under license from Biofarma. SYMPLMED and the SYMPLMED logo are trademarks of Symplmed Pharmaceutics, LLC. ACEON® is a registered trademark of Biofarma used by Symplmed Pharmaceutics, LLC under license from Biofarma and NORVASC® is a trademark of Pfizer.
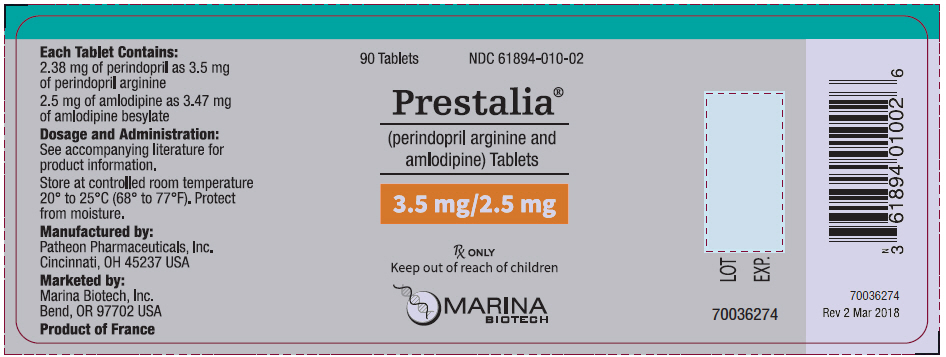
PRINCIPAL DISPLAY PANEL - 3.5 mg/2.5 mg Tablet Bottle Label
90 Tablets
NDC 61894-010-02
Prestalia®
(perindopril arginine and
amlodipine) Tablets
3.5 mg/2.5 mg
Rx ONLY
Keep out of reach of children
MARINA
BIOTECH
| PRESTALIA
perindopril arginine, amlodipine besylate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PRESTALIA
perindopril arginine, amlodipine besylate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PRESTALIA
perindopril arginine, amlodipine besylate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Marina BioTech (114131113) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Pharmaceuticals Inc. | 005286822 | MANUFACTURE(61894-010, 61894-011, 61894-012) | |