ENLON- edrophonium chloride injection, solution
Mylan Institutional LLC
----------
DESCRIPTION
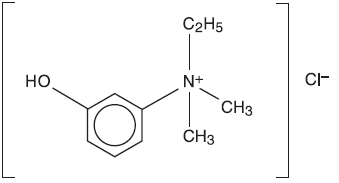
ENLON is a short and rapid-acting cholinergic drug. Chemically, edrophonium chloride is ethyl(m-hydroxyphenyl) dimethylammonium chloride and its structural formula is:
Each mL contains, in a sterile solution, 10 mg edrophonium chloride compounded with 0.45% phenol as a preservative, and 0.2% sodium sulfite as an antioxidant, buffered with sodium citrate and citric acid, and pH adjusted to approximately 5.4.
ENLON is intended for IV and IM use.
CLINICAL PHARMACOLOGY
ENLON is an anticholinesterase drug. Its pharmacologic action is due primarily to the inhibition or inactivation of acetylcholinesterase at sites of cholinergic transmission. Its effect is manifest within 30 to 60 seconds after injection and lasts an average of 10 minutes.
Pediatric Pharmacology
The pharmacology of edrophonium chloride was studied in 14 infants (between 3 weeks and 11 months old) and 12 children (between 1 year and 6 years old) during a steady-state infusion of d-tubocurarine during N2O-halothane anesthesia and controlled ventilation for elective surgery.1 The ED50 dose (dose producing 50% antagonism of 90% neuromuscular depression) for edrophonium chloride was 145 mcg/kg in infants and 233 mcg/kg in children not significantly different from that observed in adult patients; however, there was greater variability among infants and children than adults. Time to peak antagonism and duration of antagonism were similar between the two pediatric age groups and adult patients. Edrophonium chloride pharmacokinetics were studied in four infants (3 months through 7 months of age) and four children (1 through 4 years of age). Total clearance was 17.8 mL/kg•min in infants and 14.2 mL/kg•min in children. Total clearance was significantly greater in infants than in adults (8.3 + 2.9 mL/kg•min) p<0.05. Elimination half-life was 73 ± 30 minutes in infants and 99 ± 31 minutes in children compared with 126 ± 59 minutes in adult patients. Volume of distribution in infants and children was 1.18 ± 0.20 L/kg and 1.22 ± 0.74 L/kg, respectively, compared with 0.90 ± 0.13 L/kg in adults.
INDICATIONS AND USAGE
ENLON is recommended for the differential diagnosis of myasthenia gravis and as an adjunct in the evaluation of treatment requirements in this disease. It may also be used for evaluating emergency treatment in myasthenic crises. Because of its brief duration of action, it is not recommended for maintenance therapy in myasthenia gravis.
ENLON is also useful whenever a curare antagonist is needed to reverse the neuromuscular block produced by curare, tubocurarine, gallamine triethiodide or dimethyl-tubocurarine. It is not effective against decamethonium bromide and succinylcholine chloride. It may be used adjunctively in the treatment of respiratory depression caused by curare overdosage.
CONTRAINDICATIONS
Known hypersensitivity to anticholinesterase agents; intestinal and urinary obstructions of mechanical type.
WARNINGS
Whenever anticholinesterase drugs are used for testing, a syringe containing 1 mg of atropine sulfate should be immediately available to be given in aliquots intravenously to counteract severe cholinergic reactions which may occur in the hypersensitive individual, whether he is normal or myasthenic. ENLON should be used with caution in patients with bronchial asthma or cardiac dysrhythmias. The transient bradycardia which sometimes occurs can be relieved by atropine sulfate. Isolated instances of cardiac and respiratory arrest following administration of ENLON have been reported. It is postulated that these are vagotonic effects.
ENLON contains sodium sulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
PRECAUTIONS
Patients may develop “anticholinesterase insensitivity” for brief or prolonged periods. During these periods the patients should be carefully monitored and may need respiratory assistance. Dosages of anticholinesterase drugs should be reduced or withheld until patients again become sensitive to them.
Pediatric Use
The safety and effectiveness of edrophonium chloride in the differential diagnosis of myasthenia gravis have been established in pediatric patients. (See DOSAGE AND ADMINISTRATION: ENLON Test in the Differential Diagnosis of Myasthenia Gravis: Dosage in Pediatric Patients). The safety and effectiveness of edrophonium chloride in reversing neuromuscular blockade in pediatric patients have not been fully determined, although doses ranging from 0.1 mg/kg to 1.43 mg/kg have been described.2-6 Antagonism of nondepolarizing neuromuscular blocking drugs in pediatric patients is more rapid than in adults. Limited pharmacodynamic and pharmacokinetic data in pediatric patients have been published. (See CLINICAL PHARMACOLOGY: Pediatric Pharmacology.)
ADVERSE REACTIONS
Careful observation should be made for severe cholinergic reactions in the hyperreactive individual. The myasthenic patient in crisis who is being tested with ENLON should be observed for bradycardia or cardiac standstill and cholinergic reactions if an overdose is given.
The following reactions common to anticholinesterase agents may occur, although not all of these reactions have been reported with the administration of ENLON, probably because of its short duration of action and limited indications:
|
Eye: |
Increased lacrimation, pupillary constriction, spasm of accommodation, diplopia, conjunctival hyperemia. |
|
CNS: |
Convulsions, dysarthria, dysphonia, dysphagia. |
|
Respiratory: |
Increased tracheobronchial secretions, laryngospasm, bronchiolar constriction, paralysis of muscles of respiration, central respiratory paralysis. |
|
Cardiac: |
Arrhythmias (especially bradycardia), fall in cardiac output leading to hypotension. |
|
G.I.: |
Increased salivary, gastric and intestinal secretion, nausea, vomiting, increased peristalsis, diarrhea, abdominal cramps. |
|
Skeletal Muscle: |
Weakness, fasciculations. |
|
Miscellaneous: |
Increased urinary frequency and incontinence, diaphoresis. |
DRUG INTERACTIONS
Care should be given when administering this drug to patients with symptoms of myasthenic weakness who are also on anticholinesterase drugs. Since symptoms of anticholinesterase overdose (cholinergic crisis) may mimic underdosage (myasthenic weakness), their condition may be worsened by the use of this drug. (See OVERDOSAGE section for treatment.)
OVERDOSAGE
With drugs of this type, muscarine-like symptoms (nausea, vomiting, diarrhea, sweating, increased bronchial and salivary secretions and bradycardia) often appear with overdosage (cholinergic crisis). An important complication that can arise is obstruction of the airway by bronchial secretions. These may be managed with suction (especially if tracheostomy has been performed) and by the use of atropine. Many experts have advocated a wide range of dosages of atropine (for ENLON, see atropine dosage below), but if there are copious secretions, up to 1.2 mg intravenously may be given initially and repeated every 20 minutes until secretions are controlled. Signs of atropine overdosage such as dry mouth, flush and tachycardia should be avoided as tenacious secretions and bronchial plugs may form. A total dose of atropine of 5 to 10 mg or even more may be required. The following steps should be taken in the management of overdosage of ENLON:
- 1.
- Adequate respiratory exchange should be maintained by assuring an open airway and the use of assisted respiration augmented by oxygen.
- 2.
- Cardiac function should be monitored until complete stabilization has been achieved.
- 3.
- Atropine sulfate in doses of 0.4 to 0.5 mg should be administered intravenously. This may be repeated every 3 to 10 minutes. Because of the short duration of action of ENLON the total dose required will seldom exceed 2 mg.
- 4.
- If convulsions occur or shock is present, appropriate measures should be instituted.
DOSAGE AND ADMINISTRATION
ENLON Test in the Differential Diagnosis of Myasthenia Gravis7-14
Intravenous Dosage (Adults)
A tuberculin syringe containing 1 mL (10 mg) of ENLON is prepared with an intravenous needle, and 0.2 mL (2 mg) is injected intravenously within 15 to 30 seconds. The needle is left in situ. Only if no reaction occurs after 45 seconds is the remaining 0.8 mL (8 mg) injected. If a cholinergic reaction (muscarinic side effects, skeletal muscle fasciculations and increased muscle weakness) occurs after injection of 0.2 mL (2 mg), the test is discontinued and atropine sulfate, 0.4 mg to 0.5 mg, is administered intravenously. After one-half hour the test may be repeated.
Intramuscular Dosage (Adults)
In adults with inaccessible veins, dosage for intramuscular injection is 1 mL (10 mg) of ENLON. Subjects who demonstrate hyperreactivity to this injection (cholinergic reaction), should be retested after one-half hour with 0.2 mL (2 mg) of ENLON intramuscularly to rule out false-negative reactions.
Dosage in Pediatric Patients
The intravenous testing dose of ENLON in pediatric patients weighing up to 75 lbs is 0.1 mL (1 mg); above this weight, the dose is 0.2 mL (2 mg). If there is no response after 45 seconds, it may be titrated up to 0.5 mL (5 mg) in pediatric patients under 75 lbs, given in increments of 0.1 mL (1 mg) every 30 to 45 seconds and up to 1 mL (10 mg) in heavier patients. In infants, the recommended dose is 0.05 mL (0.5 mg). Because of technical difficulty with intravenous injection in pediatric patients, the intramuscular route may be used. In pediatric patients weighing up to 75 lbs, 0.2 mL (2 mg) is injected intramuscularly. In pediatric patients weighing more than 75 lbs, 0.5 mL (5 mg) is injected intramuscularly. All signs which would appear with the intravenous test appear with the intramuscular test except that there is a delay of 2 to 10 minutes before a reaction is noted.
ENLON Test for Evaluation of Treatment Requirements in Myasthenia Gravis
The recommended dose is 0.1 mL to 0.2 mL (1 mg to 2 mg) of ENLON, administered intravenously 1 hour after oral intake of the drug being used in treatment.7-11 Response will be myasthenic in the undertreated patient, adequate in the controlled patient, and cholinergic in the overtreated patient. Responses to ENLON in myasthenic and nonmyasthenic individuals are summarized in the following chart.8
|
|||
|
Myasthenic* |
Adequate† |
Cholinergic‡ |
|
|
Muscle Strength (ptosis, diplopia dysphonia, dysphagia, dysarthria, respiration, limb strength) |
Increased |
No change |
Decreased |
|
Fasciculations (orbicularis oculi, facial muscles, limb muscles) |
Absent |
Present or absent |
Present or absent |
|
Side reactions (lacrimation diaphoresis, salivation, abdominal cramps, nausea, vomiting, diarrhea) |
Absent |
Minimal |
Severe |
ENLON Test in Crisis
The term crisis is applied to the myasthenic whenever severe respiratory distress with objective ventilatory inadequacy occurs and the response to medication is not predictable. This state may be secondary to a sudden increase in severity of myasthenia gravis (myasthenic crisis), or to overtreatment with anticholinesterase drugs (cholinergic crisis).
When a patient is apneic, controlled ventilation must be secured immediately in order to avoid cardiac arrest and irreversible central nervous system damage. No attempt is made to test with ENLON until respiratory exchange is adequate.
Dosage used at this time is most important
If the patient is cholinergic, ENLON will cause increased oropharyngeal secretions and further weakness in the muscles of respiration. If the crisis is myasthenic, the test clearly improves respiration and the patient can be treated with longer-acting intravenous anticholinesterase medication. When the test is performed, there should not be more than 0.2 mL (2 mg) ENLON in the syringe. An intravenous dose of 0.1 mL (1 mg) is given initially. The patient’s heart action is carefully observed. If, after an interval of 1 minute, this dose does not further impair the patient, the remaining 0.1 mL (1 mg) can be injected. If no clear improvement of respiration occurs after 0.2 mL (2 mg) dose, it is usually wisest to discontinue all anticholinesterase drug therapy and secure controlled ventilation by tracheostomy with assisted respiration.5
For Use as a Curare Antagonist
ENLON should be administered by intravenous injection in 1 mL (10 mg) doses given slowly over a period of 30 to 45 seconds so that the onset of cholinergic reaction can be detected. This dosage may be repeated whenever necessary. The maximal dose for any one patient should be 4 mL (40 mg). Because of its brief effect, ENLON should not be given prior to the administration of curare, tubocurarine, gallamine triethiodide or dimethyl-tubocurarine: it should be used at the time when its effect is needed. When given to counteract curare overdosage, the effect of each dose on the respiration should be carefully observed before it is repeated, and assisted ventilation should always be employed.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
ENLON (edrophonium chloride injection, USP):
NDC 67457-190-15 15 mL Multiple-Dose Vial
ENLON (edrophonium chloride injection, USP)
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
REFERENCES
- 1.
- Fisher DM, Cronnelly R, Sharma M, Miller RD. Anesthesiology. 1984; 428-433.
- 2.
- Meakin G, Sweet PT, Bevan JC, Bevan DR. Neostigmine and edrophonium as antagonists of pancuronium in infants and children. Anesthesiology. 1983; 59:316-321.
- 3.
- Abdulatif M, Al-Ghamdi A, Al-Sanabary M, Abdel-Gaffar ME. Br. J. Anesth. 1996; 76:239-244.
- 4.
- Bevan JC, Tousignant C, Stephenson C, et al. Dose responses for neostigmine and edrophonium as antagonists of mivacurium in adults and children. Anesthesiology. 1996; 84:354-361.
- 5.
- Kirkegaard-Nielsen H, Meretoja OA, Wirtavuori K. Reversal of atracurium-induced neuromuscular block in paediatric patients. Acta Anesthesiol. Scand. 1995; 39:906-911.
- 6.
- Gwinnutt CL, Walker WM, Meakin G. Antagonism of intense atracurium-induced neuromuscular block in children. Br. J. Anesth. 1991; 67:13-16.
- 7.
- Osserman KE, Kaplan LI. JAMA. 1952; 150:265.
- 8.
- Osserman KE, Kaplan LI, Besson G. J Mt Sinai Hosp. 1953; 20:165.
- 9.
- Osserman KE, Kaplan LI. Arch Neurol & Psychiat. 1953; 70:385.
- 10.
- Osserman KE, Teng P. JAMA. 1956; 160:153.
- 11.
- Osserman KE, Genkins G. Ann NY Acad Sci. 1966; 135:312.
- 12.
- Tether JE. Second International Symposium Proceedings, Myasthenia Gravis. 1961:444.
- 13.
- Tether JE. In: HF Conn. Current Therapy. Philadelphia: WB Saunders Company; 1960:551.
- 14.
- Tether JE. In: HF Conn. Current Therapy. Philadelphia: WB Saunders Company; 1965:556.
Enlon is a registered trademark of Mylan Teoranta.
Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.
Manufactured by:
Akorn, Inc.
Lake Forest, IL 60045 U.S.A.
BED00N Rev. 01/13
REVISED JANUARY 2013
MI:ENLOIJ:R2
PRINCIPAL DISPLAY PANEL - 150 mg/15 mL
NDC 67457-190-15 15 mL
Enlon®
(edrophonium
chloride
injection, USP)
150 mg/15 mL
(10 mg/mL)
For Intravenous or
Intramuscular Use
Rx only Multiple-Dose Vial
Usual Dosage:
See accompanying
prescribing
information.
Store at 20° to
25°C (68° to
77°F). [See USP
Controlled Room
Temperature.]
Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.
Made in U.S.A.
MI:190:1C:R2
BEDABC Rev. 01/13
Mylan.com
Each mL contains:
edrophonium chloride
10 mg; 0.45% phenol
as a preservative;
0.2% sodium sulfite
as an antioxidant;
buffered with sodium
citrate and citric acid.
Its pH is adjusted to
approximately 5.4.
Enlon is a registered
trademark of Mylan Teoranta
| ENLON
edrophonium chloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mylan Institutional LLC (790384502) |