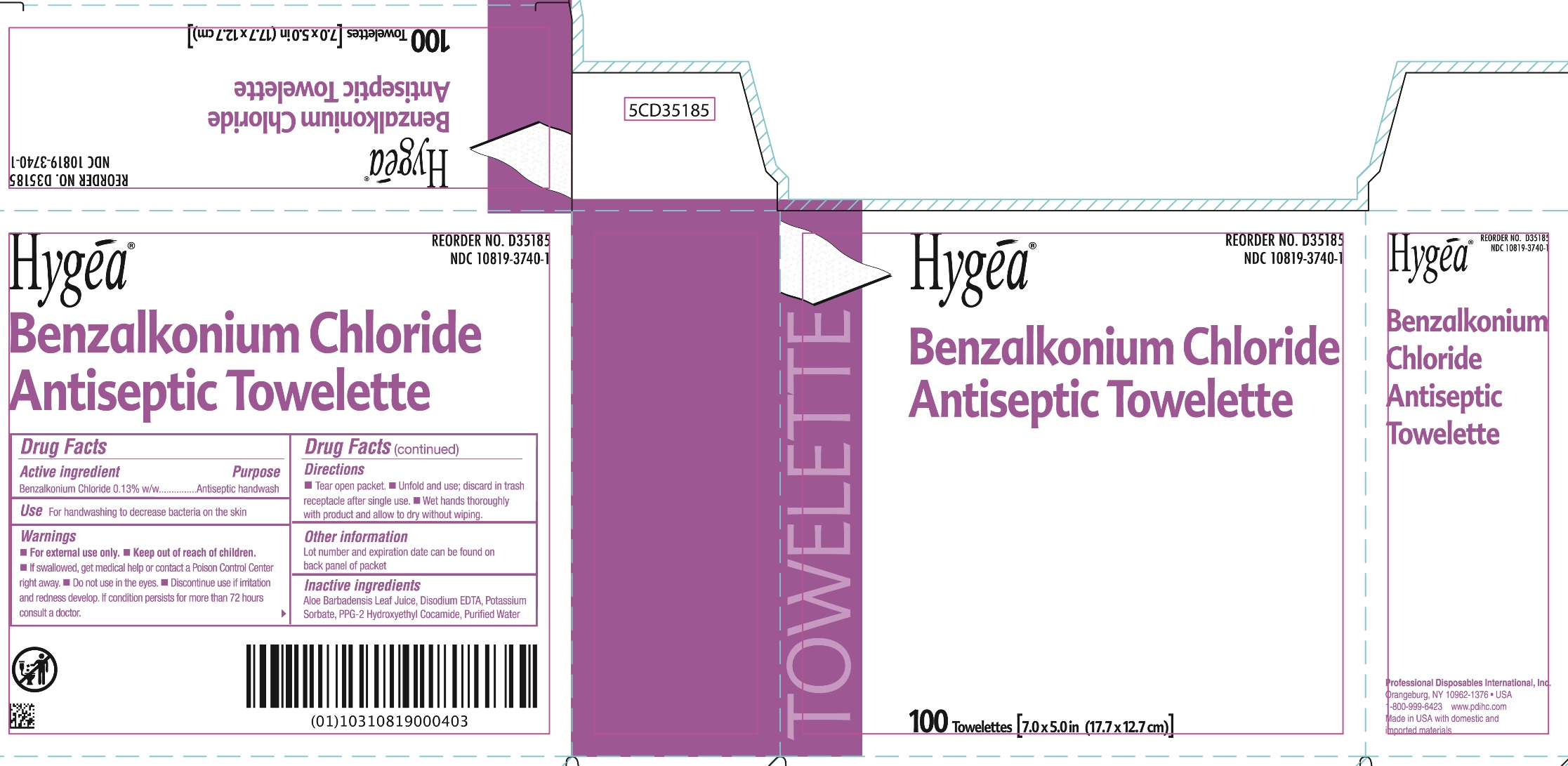
BENZALKONIUM CHLORIDE- benzalkonium chloride swab
Professional Disposables International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
HYGEA BENZALKONIUM CHLORIDE ANTISEPTIC TOWELETTE 10819-3740
WARNINGS
- For external use only.
- If swallowed, get medical help or contact a Poison Control Center right away.
- Discontinue use if irritation and redness develop. If condition persists for more than 72 hours consult a doctor.
- Do not use in the eyes.
DIRECTIONS
- Tear open packet.
- Unfold and use; discard in trash receptacle after single use.
- Wet hands thoroughly with product and allow to dry without wiping.
INACTIVE INGREDIENTS
Aloe Barbadensis Leaf Juice, Disodium EDTA, Potassium Sorbate, PPG-2 Hydroxyethyl Cocamide, Purified Water
| BENZALKONIUM CHLORIDE
benzalkonium chloride swab |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Professional Disposables International, Inc. (800777117) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Professional Disposables International, Inc. | 800777117 | manufacture(10819-3740) | |
Revised: 3/2022
Document Id: db5cdab7-aa31-3735-e053-2995a90ad37b
Set id: 980c9518-db19-418c-b32c-6c7f34225853
Version: 9
Effective Time: 20220329
Professional Disposables International, Inc.