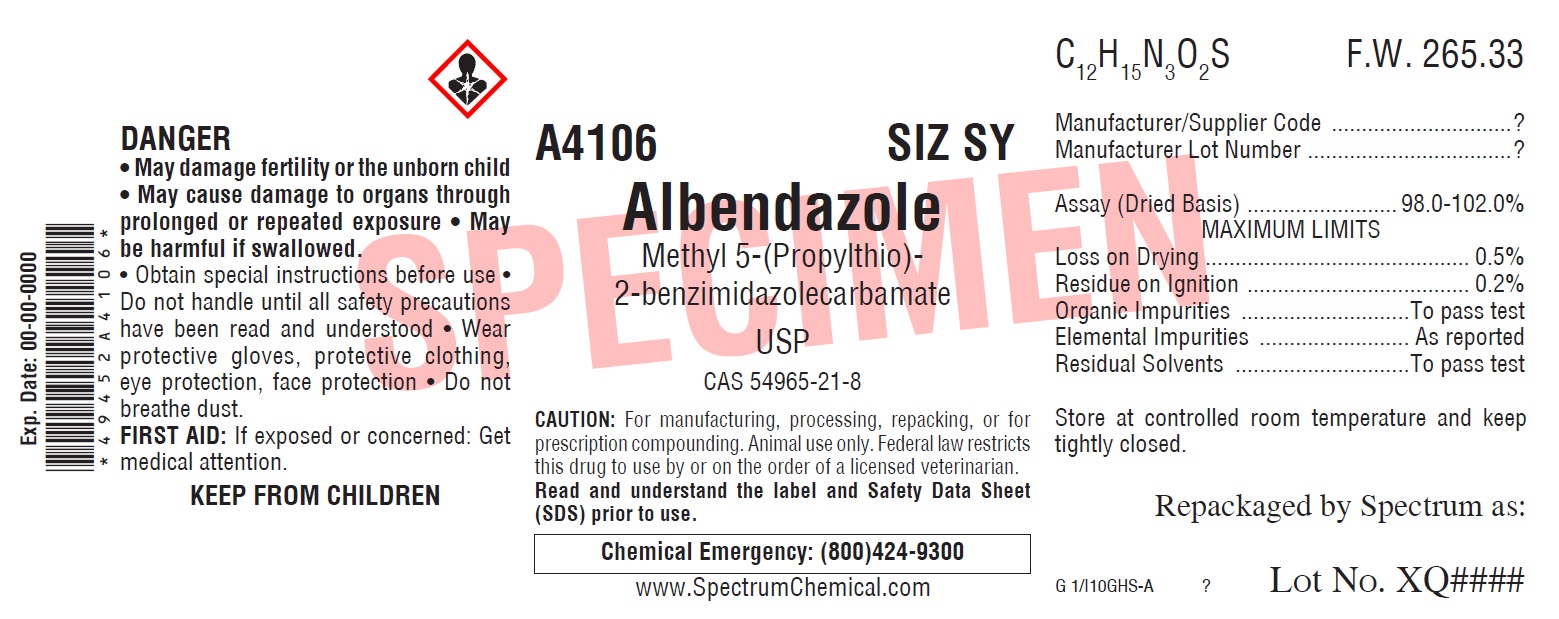
ALBENDAZOLE- albendazole powder
Spectrum Laboratory Products, Inc.
----------
Albendazole
| ALBENDAZOLE
albendazole powder |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Spectrum Laboratory Products, Inc. (075295246) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Spectrum Laboratory Products, Inc | 829407225 | relabel(49452-0026) , repack(49452-0026) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Spectrum Laboratory Products, Inc. | 962389607 | relabel(49452-0026) , repack(49452-0026) | |
Revised: 11/2021
Document Id: d1f64195-10b9-75f2-e053-2a95a90a4812
Set id: 97a61a3b-ef09-a05b-e053-2a95a90a2e2f
Version: 4
Effective Time: 20211129
Spectrum Laboratory Products, Inc.