MAGNESIUM HYDROXIDE/ALUMINUM HYDROXICE/SIMETHICONE- magnesium hydroxide,aluminum hydroxice,simethicone suspension
MAGNESIUM HYDROXIDE/ALUMINUM HYDROXICE/SIMETHICONE- magnesium hydroxide/aluminum hydroxice/simethicone suspension
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
MgOH-Al-OH-Simethicone
Major Pharmaceuticals
Unit dose OTC Monograph drugs
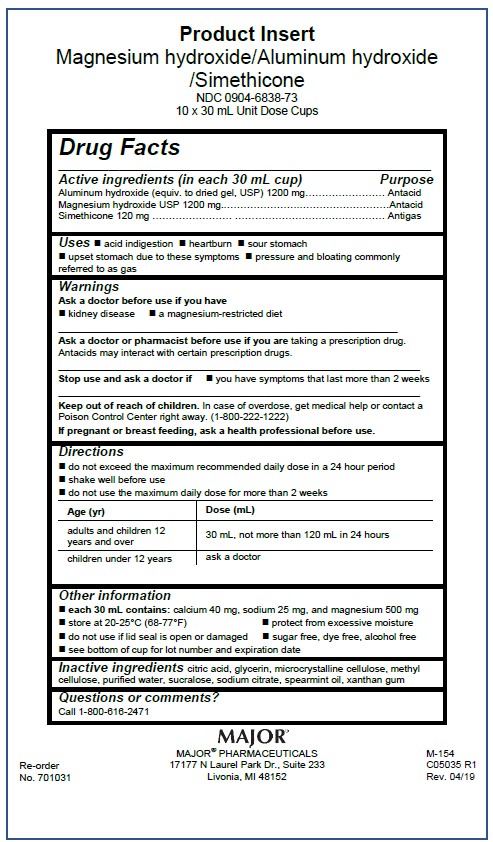
Magnesium hydroxide, Aluminum hydroxide, Simethicone 1200/1200/120 mg per 30 mL
Major Pharmaceutical OTC Monograph
 NDC 0904-6838-73
NDC 0904-6838-73
Each 30 mL Contains:
Magnesium Hydroxide1200 mg
Aluminum Hydroxide 1200 mg
Simethicone 120 mg
Delivers 30 mL
Shake Well
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Directions
- do not exceed the maximum recommended daily dose in a 24 hour period
- shake well before use
- do not use the maximum daily dose for more than 2 weeks
|
Age (yr) |
Dose (mL) |
|
adults and children 12 years and over |
30 mL, not more than 120 mL in 24 hours |
|
children under 12 years |
ask a doctor |
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium-restricted diet
___________________________________________________________________
Ask a doctor or pharmacist before use if you are taking a presecription drug. Antacids may interact with certain prescription drugs.
___________________________________________________________________
Stop use and ask a doctor if
- you have symptoms that last more than 2 weeks
___________________________________________________________________
If pregnant or breast-feeding, ask a health professional before use.
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Inactive ingredients citric acid, glycerin, microcrystalline cellulose, methyl cellulose, purified water, saccharin sodium, sodium citrate, spearmint oil, xantham gum
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Uses
- acid indigestion
- heartburn
- sour stomach
- upset stomach due to these symptoms
- pressure and bloating commonly referred to as gas
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Active ingredient (in each 30 mL cup)
Magnesium hydroxide USP 1200 mg
Magnesium hydroxide USP 1200 mg
Simethicone 120 mg
MgOH, AlOH, Simethicone 1200/1200/120 mg/30 mL
Other information
- each 30 mL contains: calcium 40 mg, sodium 25 mg, and magnesium 500 mg
- store at 20-25°C (68-77°F)
- protect from excessive moisture
- do not use if lid seal is open or damaged
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Major Pharmaceutical OTC Monograph
 NDC 0904-6839-73
NDC 0904-6839-73
Each 30 mL Contains:
Magnesium Hydroxide 2400 mg
Aluminum Hydroxide 2400 mg
Simethicone 240 mg
Max
Delivers 30 mL
Shake Well
See Insert
For Instituional Use Only
MAJOR PHARMACEUTICALS
Livonia, MI 64152
Sugar Free - Dye Free - Alcohol Free
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Directions
- do not exceed the maximum recommended daily dose in a 24 hour period
- shake well before use
- do not use the maximum daily dose for more than 2 weeks
|
Age (yr) |
Dose (mL) |
|
adults and children 12 years and over |
30 mL, not more than 60 mL in 24 hrs. |
|
children under 12 years |
ask a doctor |
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium-restricted diet
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
___________________________________________________________________
Stop use and ask a doctor if
- you have symptoms that last for more than 2 weeks.
___________________________________________________________________
If pregnant or breast-feeding, ask a health professional before use.
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Inactive ingredients citric acid, glycerin, microcrystalline cellulose, methyl cellulose, purified water, saccharin sodium, sodium citrate, spearmint oil, xanthan gum
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Uses
- acid indigestion
- heartburn
- sour stomach
- upset stomach due to these symptoms
- pressure and bloating commonly referred to as gas
Magnesium hydroxide, Aluminum hydroxide, Simethicone 2400/2400/240 mg/30 mL
Other information
- each 30 mL contains: calcium 40 mg, sodium 25 mg, and magnesium 1000 mg
- store at 20-25°C (68-77°F) -
- protect from excessive moisture
- do not use if lid seal is open or damaged -
- sugar free, dye free, alcohol free
- see bottom of cup for lot number and expiration date
| MAGNESIUM HYDROXIDE/ALUMINUM HYDROXICE/SIMETHICONE
magnesium hydroxide,aluminum hydroxice,simethicone suspension |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| MAGNESIUM HYDROXIDE/ALUMINUM HYDROXICE/SIMETHICONE
magnesium hydroxide/aluminum hydroxice/simethicone suspension |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
| Registrant - Plastikon Healthcare, LLC (041717941) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Plastikon Healthcare, LLC | 041717941 | manufacture(0904-6838, 0904-6839) | |