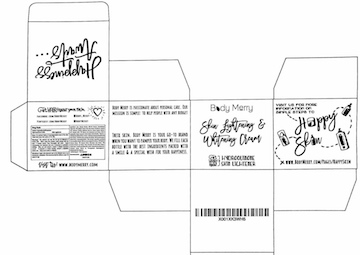
BODY MERRY LIGHTENING AND WHITENING CREAM- hydroquinone cream
Body Merry, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients/Purpose:
Hydroquinone 2.0% Skin Lightener
Active Ingredient/Purpose:
Skin Lightener
Warnings:
For external use only. Do not use on inflammed or broken skin. When using this product, avoid contact with the eyes. If contact occurs, rinse thoroughly with water. Avoid unnecessary sun exposure and use a sunscreen or protective clothing. Stop use and consult a doctor if irritation becomes severe. If pregnant or breastfeeding, consult a physician before use. Keep out of reach of children.
Uses
For gradual fading of hyperpigmented areas of the skin. Helps fade away dark spots on the skin.
Directions:
Apply onto cleansed skin of the affected area or as directed by a physician. Not recommended for children under 12 years of age.
Inactive Ingredients:
water, glyceryl streate, PEG-100 stearate, stearic acid
Warning
Keep out of reach of children.