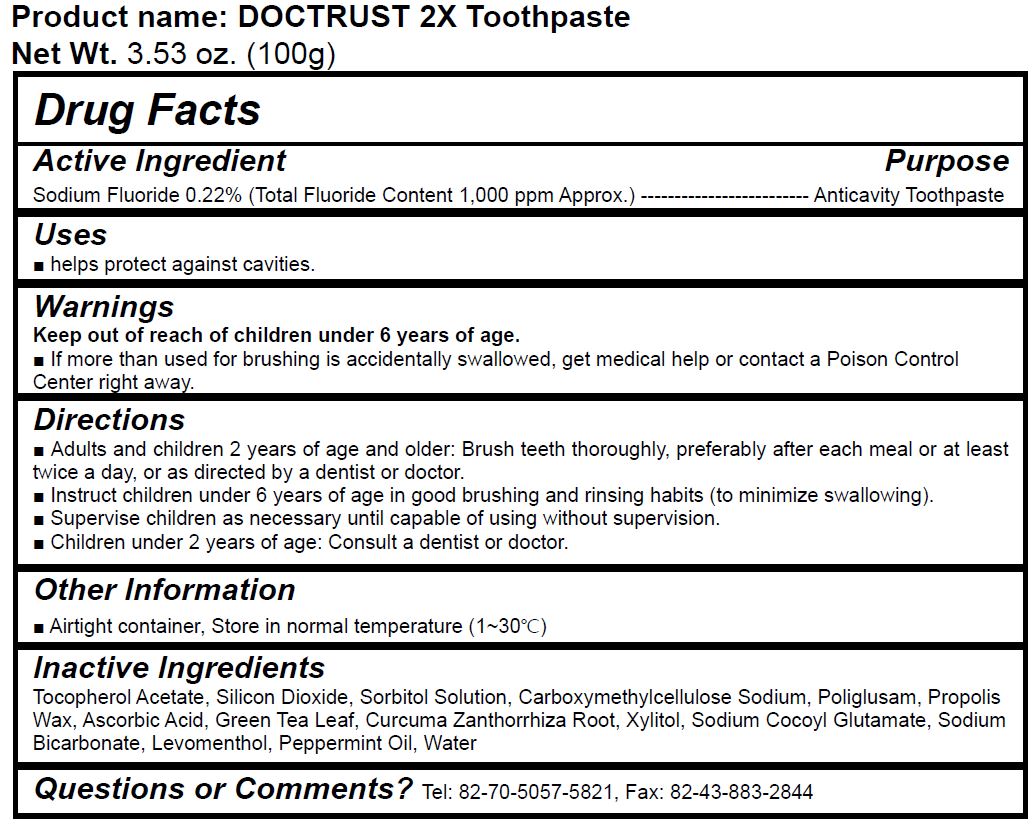
DOCTRUST 2X TOOTH- sodium fluoride paste
DONG IL PHARMS CO.,LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Keep your teeth white and strong.
Keep your mouth clean.
Refresh the inside of the mouth.
Prevents tooth decay and bad breath by fluoride
Increase esthetic effect.
Removal of plaque (anti-plaque)
Prevention of gingivitis and periodontitis
Periodontal Disease Prevention
Prevention of gum disease
Keep out of reach of children
Brushing the teeth with a suitable amount
(1) Contains 1000ppm of fluoride.
(2) Do not swallow and rinse mouth thoroughly after use
(3) If you experience any problems with your gums or mouth during use,
discontinue use and consult your doctor.
(4) For children under 6 years of age, use small amounts of toothpaste. And
use itunder the supervision of a guardian to avoid sucking or swallowing.
(5) Consult a physician or dentist immediately if a child under 6 years old
hasswallowed large quantities.
(6) Keep out of the reach of children under 6 years of age.
Tocopherol Acetate, Silicon Dioxide, D-Sorbitol Solution, Carboxymethylcellulose Sodium, Chitosan, Propolis Extract, Glycyrrhiza Extract Powder, Ascorbic Acid, Green Tea Extract, Curcuma xanthorrhiza Extract, Xylitol, Enzymatically Modified Stevia, Sodium Cocoyl Glutamate, Sodium Bicarbonate, L-Menthol, Peppermint Oil, Water