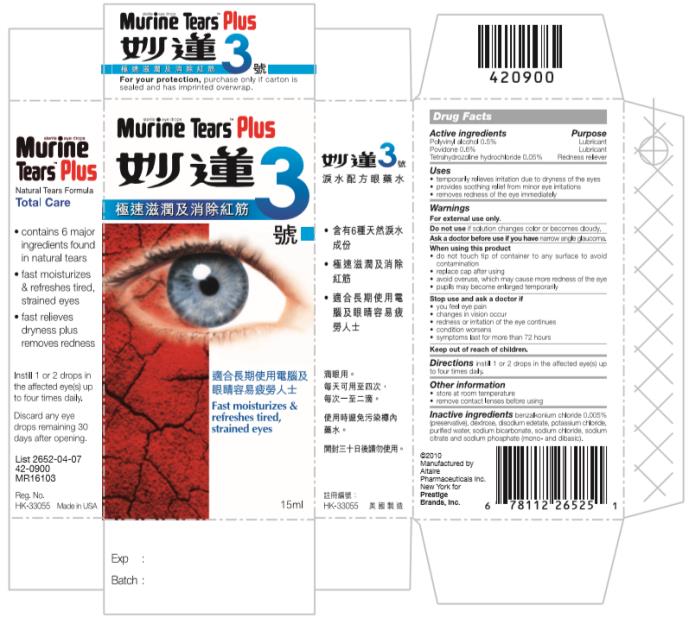
MURINE TEARS PLUS 3- polyvinyl alcohol, povidone and tetrahydrozoline hydrochloride liquid
Prestige Brands Holdings, Inc.
----------
Murine Tears Plus 3 - Total Care
Uses
- temporarily relieves irritation due to dryness of the eye
- provides soothing relief from minor eye irritations
- removes redness from the eye
Warnings
For external use only.
When using this product
- do not touch tip to any surface to avoid contamination
- replace cap after using
- avoid overuse, which may cause more redness of the eye
- pupils may become enlarged temporarily
| MURINE TEARS PLUS 3
polyvinyl alcohol, povidone and tetrahydrozoline hydrochloride liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Prestige Brands Holdings, Inc. (159655021) |
Revised: 11/2023
Document Id: ad30d032-5cf9-4aba-b426-cc4b40d05c96
Set id: 97181be2-1e4e-40d9-819a-7ec7e49013be
Version: 3
Effective Time: 20231113
Prestige Brands Holdings, Inc.