DOCUSATE CALCIUM- docusate calcium capsule
NCS HealthCare of KY, LLC dba Vangard Labs
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Docusate Calcium
Uses
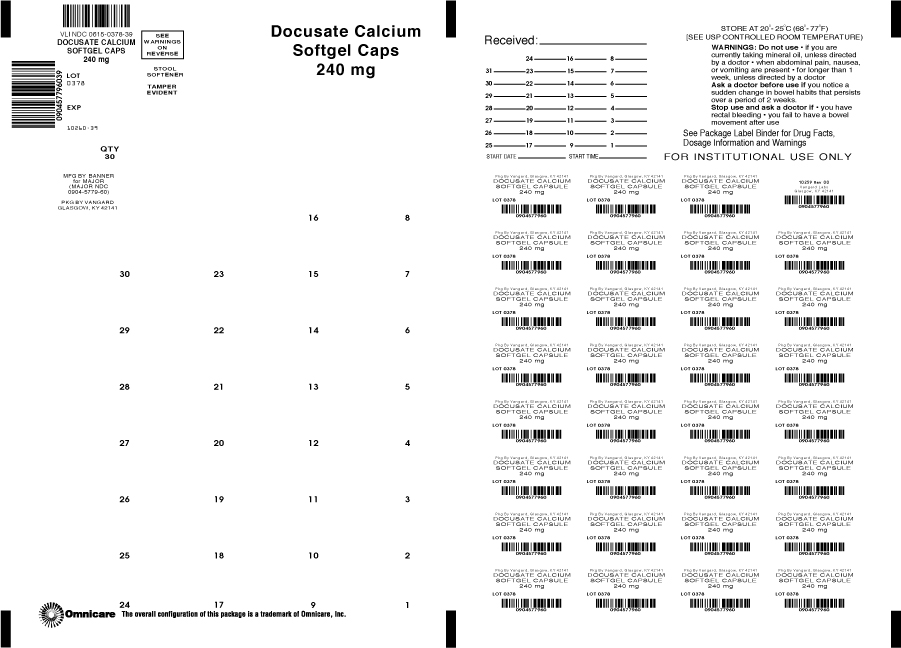
For relief of occasional constipation. This product generally produces a bowel movement within 12 to 72 hours.
Warnings
Do not use
-if you are currently taking mineral oil, unless directed by a doctor
-when abdominal pain, nausea, or vomiting are present
-for longer than 1 week, unless directed by a doctor
Directions
-adults and children over 12 years of age: take 1 softgel daily for several days, or until bowel movements are normal, or as directed by a doctor
-children under 12 years of age: take as directed by a doctor
Other information
-store at controlled room temperature 15º - 30ºC (59º - 86ºF)
-*This product is not manufactured or distributed by Chattem, Inc., owner of the registered trademark Surfak® Stool Softener.
Inactive ingredients
Corn oil, D&C Red #33, edible white ink, FD&C Red #40, gelatin, glycerin, purified water and sorbitol special.
| DOCUSATE CALCIUM
docusate calcium capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - NCS HealthCare of KY, LLC dba Vangard Labs (050052943) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NCS HealthCare of KY, LLC dba Vangard Labs | 050052943 | repack(0615-0378) | |