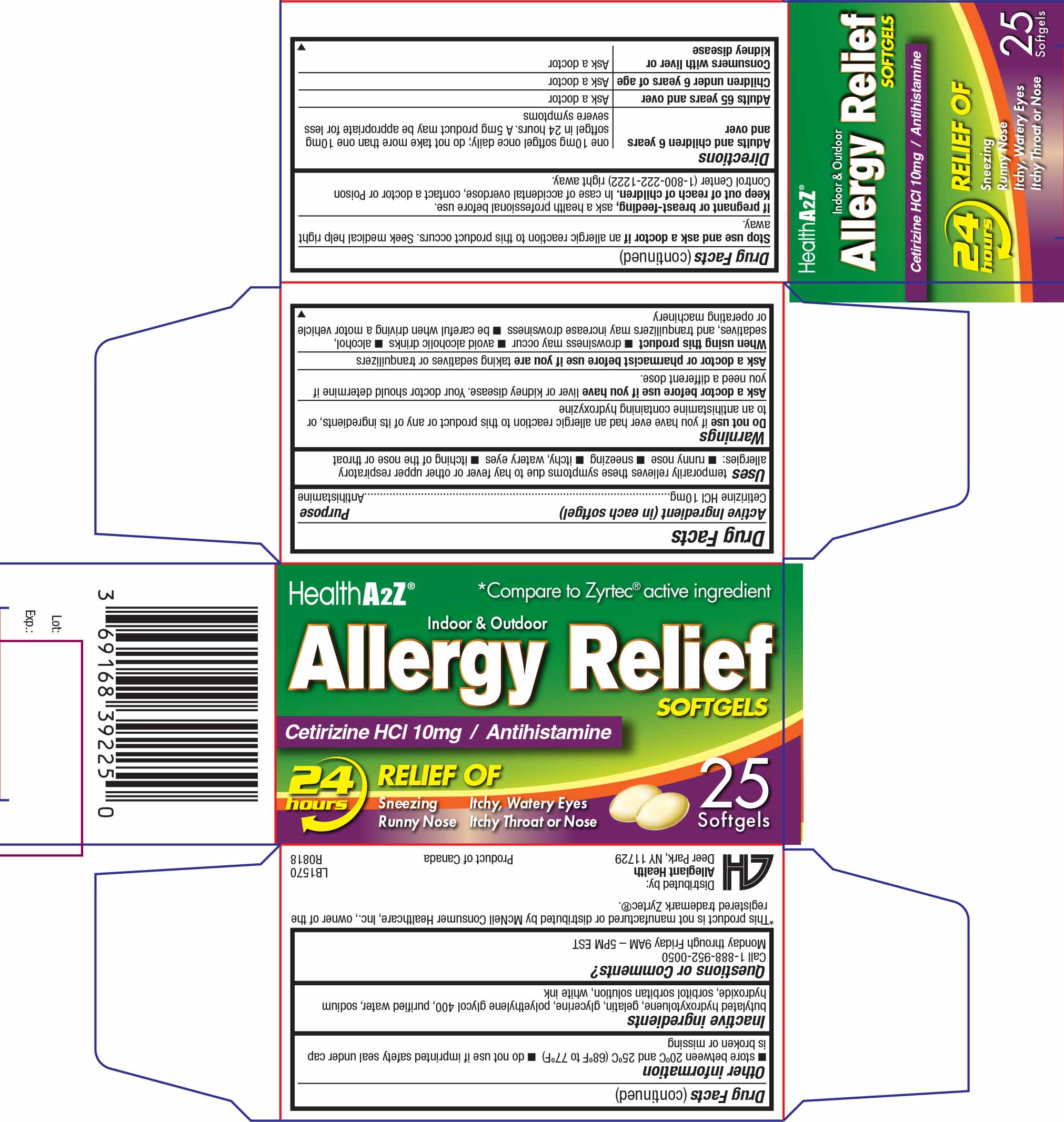
Label: ALLERGY RELIEF- cetirizine hcl 10 mg capsule, liquid filled
- NDC Code(s): 69168-392-25
- Packager: Allegiant Health
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 31, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each softgel)
- Purpose
- Uses
-
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when drivinga motor vehicle or operating machinery
- Keep Out of Reach of Children
-
Directions
- Adults and children 6 years and over: one 10mg tablet once daily; do not take more than one 10mg tablet in 24 hours. A 5mg product may be appropriate for less severe symptoms
- Adults 65 years and over: ask a doctor
- Children under 6 years of age: ask a doctor
- Consumers with liver or kidney disease: ask a doctor
- Other information
- Inactive Ingredients
- Questions or Comments?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
cetirizine hcl 10 mg capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69168-392 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color YELLOW Score no score Shape OVAL Size 14mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69168-392-25 1 in 1 CARTON 12/16/2014 1 25 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078317 12/16/2014 Labeler - Allegiant Health (079501930)