Label: WAL-FEX- fexofenadine hydrochloride tablet
-
NDC Code(s):
0363-0784-07,
0363-0784-15,
0363-0784-18,
0363-0784-29, view more0363-0784-30, 0363-0784-40, 0363-0784-43, 0363-0784-45, 0363-0784-75, 0363-0784-90
- Packager: Walgreens Company
- This is a repackaged label.
- Source NDC Code(s): 55111-784
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 22, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
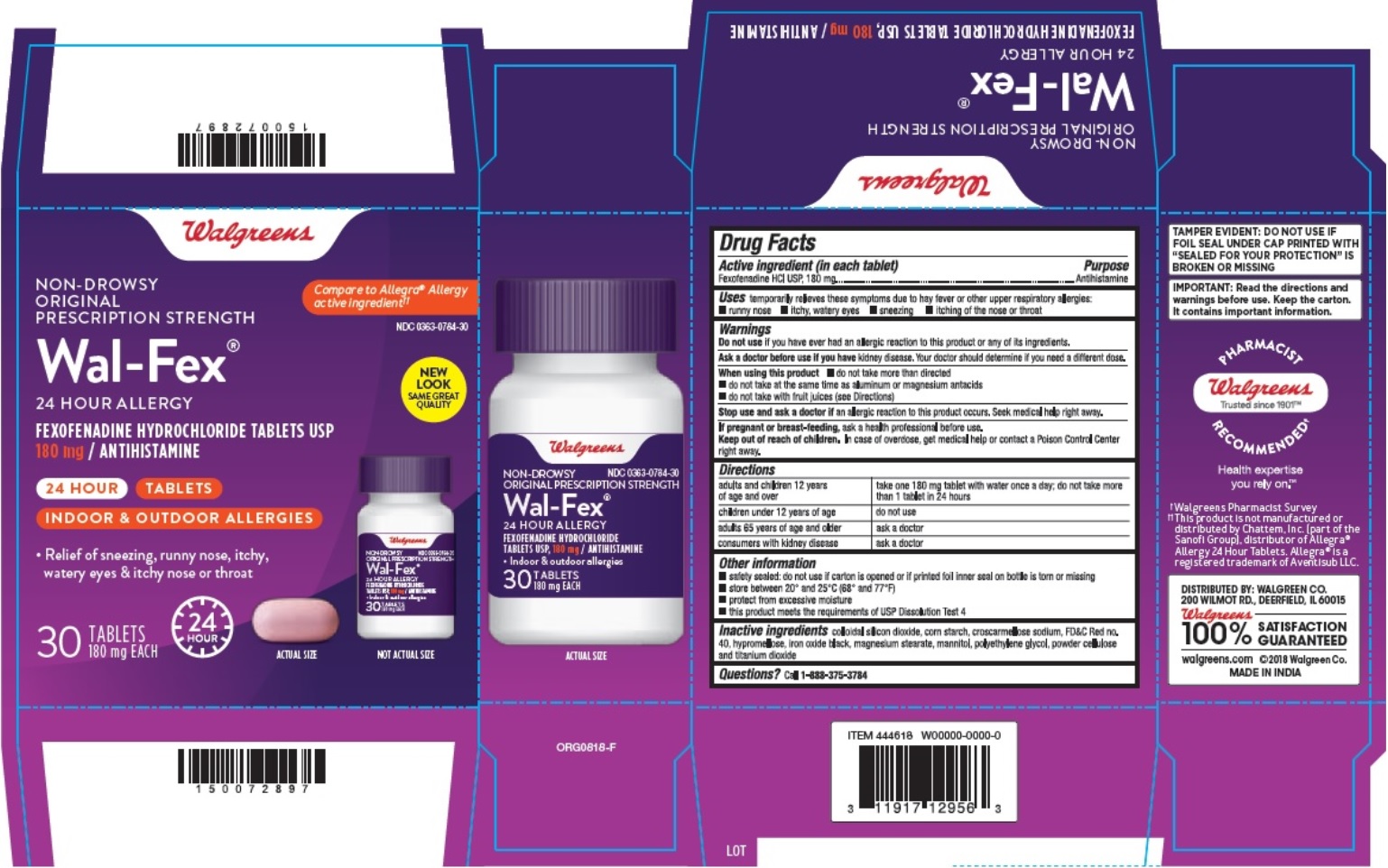
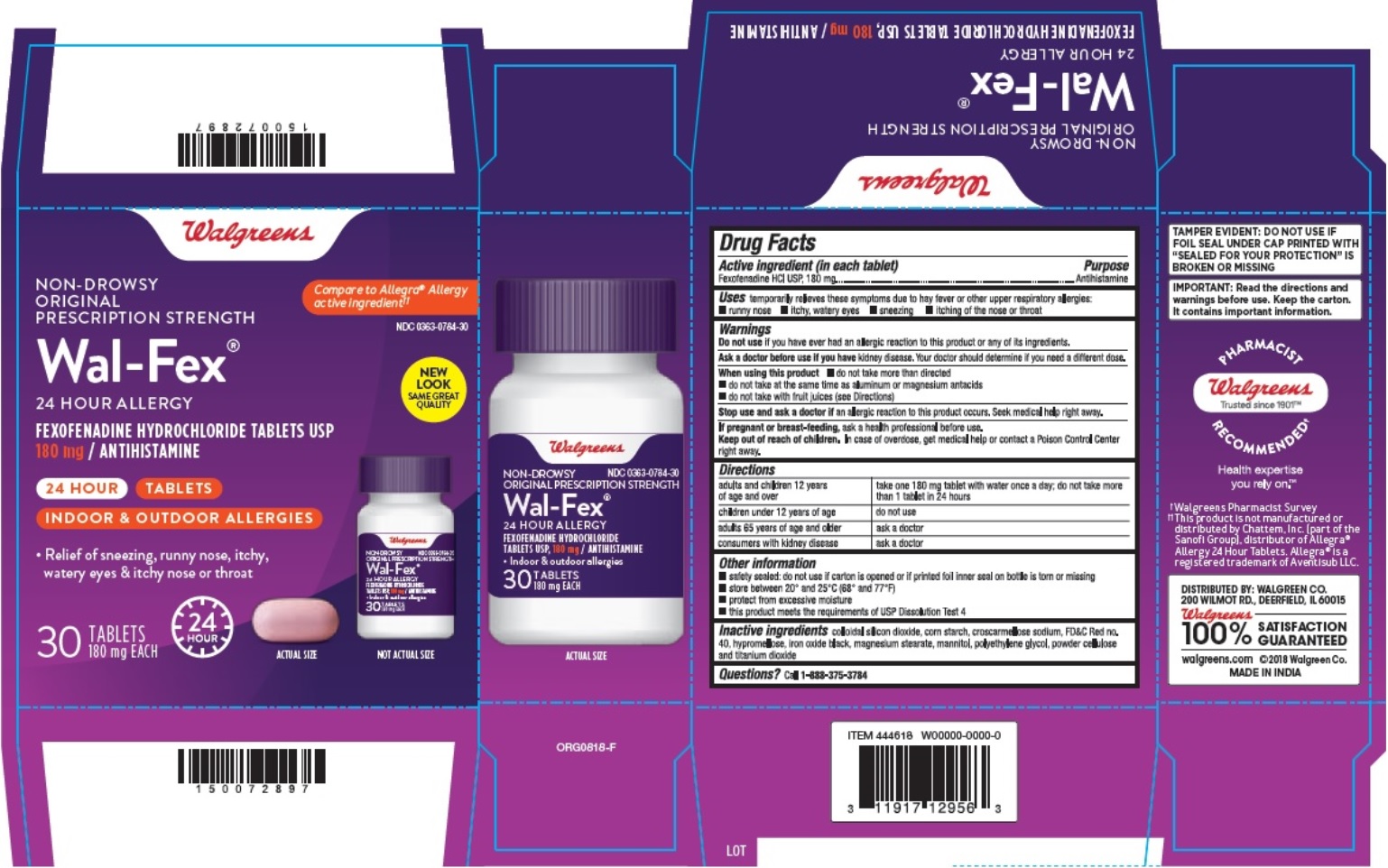
- Active ingredient(s)
- Purpose
- Use(s)
-
Warnings
Ask a doctor before use if you have
kidney disease.Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- Directions
- Other information
- Storage
- Inactive ingredients
- Questions?
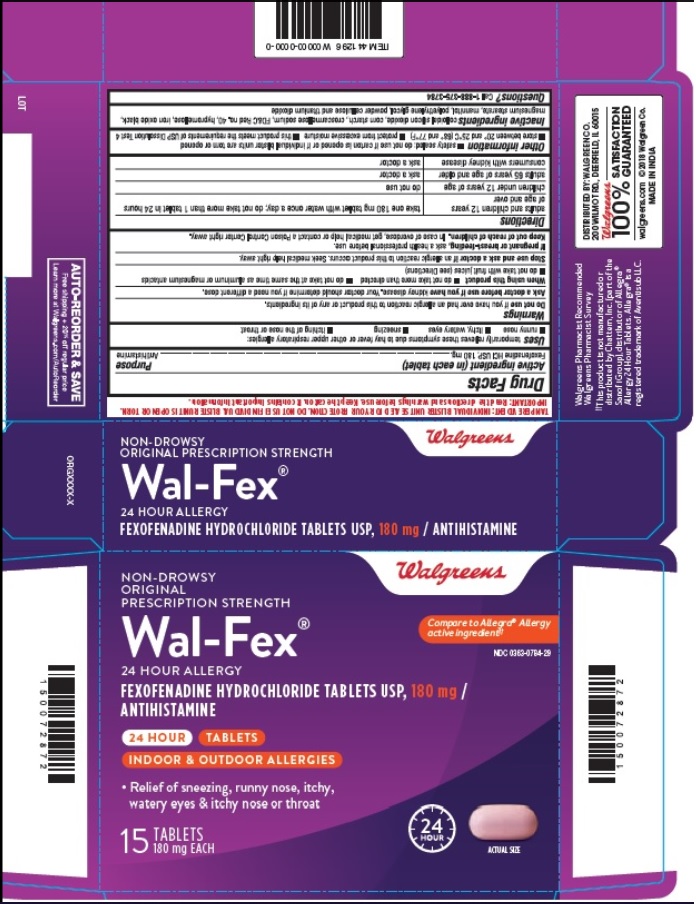
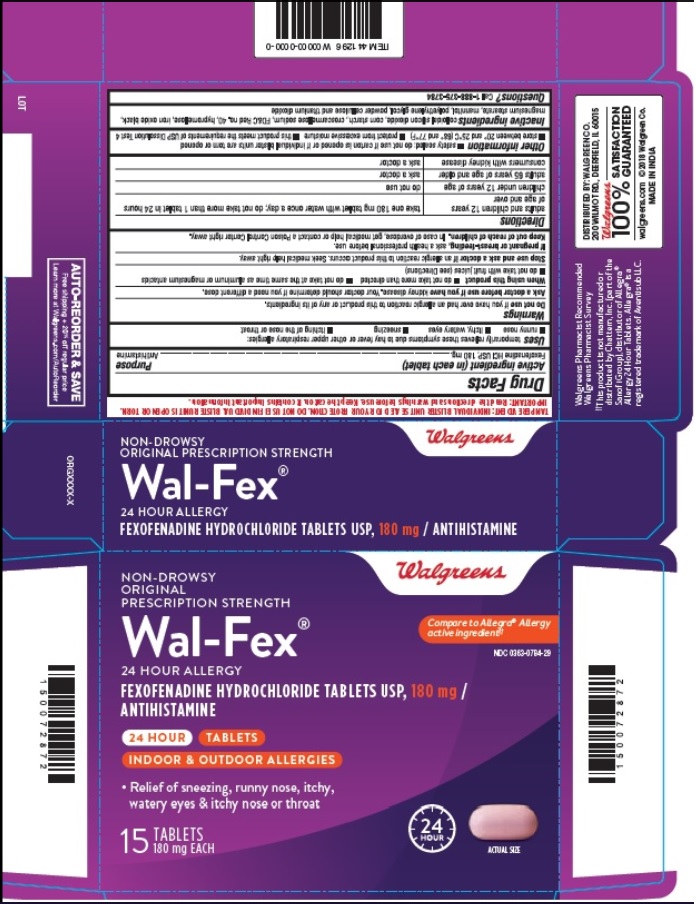
- PRINCIPAL DISPLAY PANEL
- Blister Carton Label: 15 count
-
INGREDIENTS AND APPEARANCE
WAL-FEX
fexofenadine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-0784(NDC:55111-784) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fexofenadine Hydrochloride (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) Fexofenadine Hydrochloride 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) magnesium stearate (UNII: 70097M6I30) mannitol (UNII: 3OWL53L36A) POWDERED CELLULOSE (UNII: SMD1X3XO9M) FD&C RED NO. 40 (UNII: WZB9127XOA) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) FERROSOFERRIC OXIDE (UNII: XM0M87F357) polyethylene glycol 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color PINK Score no score Shape OVAL Size 7mm Flavor Imprint Code 194;R Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-0784-30 1 in 1 CARTON 04/13/2011 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0363-0784-43 2 in 1 CARTON 04/13/2011 2 30 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:0363-0784-40 1 in 1 CARTON 04/13/2011 3 40 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:0363-0784-45 1 in 1 CARTON 04/13/2011 4 45 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:0363-0784-75 1 in 1 CARTON 04/13/2011 5 70 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:0363-0784-15 1 in 1 CARTON 04/13/2011 6 150 in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC:0363-0784-90 1 in 1 CARTON 04/13/2011 7 90 in 1 BOTTLE; Type 0: Not a Combination Product 8 NDC:0363-0784-07 1 in 1 CARTON 04/13/2011 8 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 9 NDC:0363-0784-29 3 in 1 CARTON 04/13/2011 9 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 10 NDC:0363-0784-18 180 in 1 BOTTLE; Type 0: Not a Combination Product 07/13/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076502 04/13/2011 Labeler - Walgreens Company (008965063)