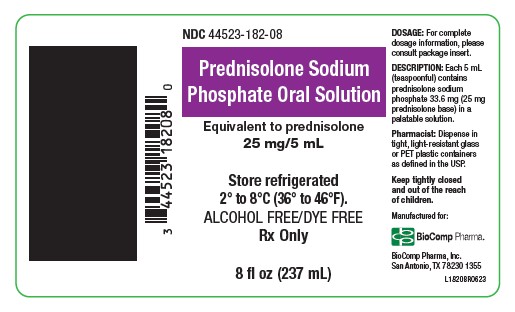
Label: PREDNISOLONE SODIUM PHOSPHATE ORAL SOLUTION- prednisolone sodium phosphate solution
- NDC Code(s): 44523-182-08
- Packager: BioComp Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated March 28, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) is a dye free, pale to light yellow solution. Each 5 mL (teaspoonful) of prednisolone sodium phosphate oral solution contains 33.6 mg prednisolone sodium phosphate (25 mg prednisolone base) in a palatable, aqueous vehicle.
Prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) also contains antibitter mask, corn syrup, edetate disodium, glycerin, grape flavor, hydroxyethylcellulose, methylparaben, potassium phosphate dibasic, potassium phosphate monobasic, purified water, and sodium saccharin.
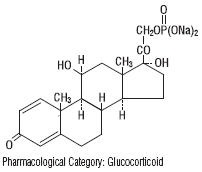
Prednisolone sodium phosphate occurs as white or slightly yellow, friable granules or powder. It is freely soluble in water; soluble in methanol; slightly soluble in alcohol and in chloroform; and very slightly soluble in acetone and in dioxane. The chemical name of prednisolone sodium phosphate is pregna-1,4-diene-3,20- dione,11,17-dihydroxy- 21-(phosphonooxy)- disodium salt, (11ß)-. The empirical formula is C 21H 27Na 2O 8P; the molecular weight is 484.39. Its chemical structure is:
-
CLINICAL PHARMACOLOGY
Naturally occurring glucocorticoids (hydrocortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs are primarily used for their potent anti-inflammatory effects in disorders of many organ systems.
Prednisolone is a synthetic adrenocortical steroid drug with predominantly glucocorticoid properties. Some of these properties reproduce the physiological actions of endogenous glucocorticosteroids, but others do not necessarily reflect any of the adrenal hormones’ normal functions; they are seen only after administration of large therapeutic doses of the drug. The pharmacological effects of prednisolone which are due to its glucocorticoid properties include: promotion of gluconeogenesis; increased deposition of glycogen in the liver; inhibition of the utilization of glucose; anti-insulin activity; increased catabolism of protein; increased lipolysis; stimulation of fat synthesis and storage; increased glomerular filtration rate and resulting increase in urinary excretion of urate (creatinine excretion remains unchanged); and increased calcium excretion.
Depressed production of eosinophils and lymphocytes occurs, but erythropoiesis and production of polymorphonuclear leukocytes are stimulated. Inflammatory processes (edema, fibrin deposition, capillary dilatation, migration of leukocytes and phagocytosis) and the later stages of wound healing (capillary proliferation, deposition of collagen, cicatrization) are inhibited.
Prednisolone can stimulate secretion of various components of gastric juice. Suppression of the production of corticotropin may lead to suppression of endogenous corticosteroids. Prednisolone has slight mineralocorticoid activity, whereby entry of sodium into cells and loss of intracellular potassium is stimulated. This is particularly evident in the kidney, where rapid ion exchange leads to sodium retention and hypertension.
Prednisolone is rapidly and well absorbed from the gastrointestinal tract following oral administration.
Prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) produces a 14% higher peak plasma level of prednisolone which occurs 20% faster than that seen with tablets. Prednisolone is 70-90% protein-bound in the plasma, and it is eliminated from the plasma with a half-life of 2 to 4 hours. It is metabolized mainly in the liver and excreted in the urine as sulfate and glucuronide conjugates.
The systemic availability, metabolism and elimination of prednisolone after administration of single weight-based doses (0.8 mg/kg) of intravenous (IV) prednisolone and oral prednisone were reported in a small study of 19 young (23 to 34 years) and 12 elderly (65 to 89 years) subjects. Results showed that the systemic availability of total and unbound prednisolone, as well as interconversion between prednisolone and prednisone were independent of age. The mean unbound fraction of prednisolone was higher, and steady-state volume of distribution (Vss) of unbound prednisolone was reduced in elderly patients. Plasma prednisolone concentrations were higher in elderly subjects, and the higher AUCs of total and unbound prednisolone were most likely reflective of an impaired metabolic clearance, evidenced by reduced fractional urinary clearance of 6ß -hydroxyprednisolone. Despite these findings of higher total and unbound prednisolone concentrations, elderly subjects had higher AUCs of cortisol, suggesting that the elderly population is less sensitive to suppression of endogenous cortisol or their capacity for hepatic inactivation of cortisol is diminished.
-
INDICATIONS AND USAGE
Prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) is indicated in the following conditions:
1. Allergic States
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in adult and pediatric populations with: seasonal or perennial allergic rhinitis; asthma; contact dermatitis; atopic dermatitis; serum sickness; drug hypersensitivity reactions.
2. Dermatologic Diseases
Pemphigus; bullous dermatitis herpetiformis; severe erythema multiforme (Stevens-Johnson syndrome); exfoliative erythroderma; mycosis fungoides.
3. Edematous States
To induce diuresis or remission of proteinuria in nephrotic syndrome in adults with lupus erythematosus and in adults and pediatric populations, with idiopathic nephritic syndrome, without uremia.
4. Endocrine Disorders
Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice; synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy mineralocorticoid supplementation is of particular importance); congenital adrenal hyperplasia; hypercalcemia associated with cancer; nonsuppurative thyroiditis.
5. Gastrointestinal Diseases
To tide the patient over a critical period of the disease in: ulcerative colitis; regional enteritis.
6. Hematologic Disorders
Idiopathic thrombocytopenic purpura in adults; selected cases of secondary thrombocytopenia; acquired (autoimmune) hemolytic anemia; pure red cell aplasia; Diamond-Blackfan anemia.
7. Neoplastic Diseases
For the treatment of acute leukemia and aggressive lymphomas in adults and children.
8. Nervous System
Acute exacerbations of multiple sclerosis.
9. Ophthalmic Diseases
Uveitis and ocular inflammatory conditions unresponsive to topical corticosteroids; temporal arteritis; sympathetic ophthalmia.10. Respiratory Diseases
Symptomatic sarcoidosis; idiopathic eosinophilic pneumonias; fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy; asthma (as distinct from allergic asthma listed above under “Allergic States”), hypersensitivity pneumonitis, idiopathic pulmonary fibrosis, acute exacerbations of chronic obstructive pulmonary disease (COPD), and Pneumocystis carinii pneumonia (PCP) associated with hypoxemia occurring in an HIV (+) individual who is also under treatment with appropriate anti-PCP antibiotics. Studies support the efficacy of systemic corticosteroids for the
treatment of these conditions: allergic bronchopulmonary aspergillosis, idiopathic bronchiolitis obliterans with organizing pneumonia.
11. Rheumatic Disorders
As adjunctive therapy for short term administration (to tide the patient over an acute episode or exacerbation) in: psoriatic arthritis; rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low dose maintenance therapy); ankylosing spondylitis; acute and subacute bursitis; acute nonspecific tenosynovitis; acute gouty arthritis; epicondylitis. For the treatment of systemic lupus erythematosus, dermatomyositis (polymyositis), polymyalgia rheumatica, Sjogren’s syndrome, relapsing polychondritis, and certain cases of vasculitis.12. Miscellaneous
Tuberculous meningitis with subarachnoid block or impending block, tuberculosis with enlarged mediastinal lymph nodes causing respiratory difficulty, and tuberculosis with pleural or pericardial effusion (appropriate antituberculous chemotherapy must be used concurrently when treating any tuberculosis complications); trichinosis with neurologic or myocardial involvement; acute or
chronic solid organ rejection (with or without other agents). - CONTRAINDICATIONS
-
WARNINGS
General: In patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during and after the stressful situation is indicated.
Immunosuppression and Increased Risk of Infection: Corticosteroids, including prednisolone sodium phosphate oral solution, suppress the immune system and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic pathogens. Corticosteroids can:
• Reduce resistance to new infections
• Exacerbate existing infections
• Increase the risk of disseminated infections
• Increase the risk of reactivation or exacerbation of latent infections
• Mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe and at times fatal. The rate of infectious complications increases with increasing corticosteroid dosages.
Monitor for the development of infection and consider prednisolone sodium phosphate oral solution withdrawal or dosage reduction as needed.
Tuberculosis: If prednisolone sodium phosphate oral solution is used to treat a condition in patients with latent tuberculosis or tuberculin reactivity, reactivation of tuberculosis may occur. Closely monitor such patients for reactivation. During prolonged prednisolone sodium phosphate oral solution therapy, patients with latent tuberculosis or tuberculin reactivity should receive chemoprophylaxis.
Varicella Zoster and Measles Viral Infections: Varicella and measles can have a serious or even fatal course in non-immune patients taking corticosteroids, including prednisolone sodium phosphate oral solution. In corticosteroid-treated patients who have not had these diseases or are non-immune, particular care should be taken to avoid exposure to varicella and measles:
• If a prednisolone sodium phosphate oral solution-treated patient is exposed to varicella, prophylaxis with varicella zoster immune globulin may be indicated. If varicella develops, treatment with antiviral agents may be considered.
• If a prednisolone sodium phosphate oral solution-treated patient is exposed to measles, prophylaxis with immunoglobulin may be indicated.
Hepatitis B Virus Reactivation: Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids, including prednisolone sodium phosphate oral solution. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection.
Screen patients for hepatitis B infection before initiating immunosuppressive (e.g., prolonged) treatment with prednisolone sodium phosphate oral solution. For patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Fungal Infections: Corticosteroids, including prednisolone sodium phosphate oral solution, may exacerbate systemic fungal infections; therefore, avoid prednisolone sodium phosphate oral solution use in the presence of such infections unless prednisolone sodium phosphate oral solution is needed to control drug reactions. For patients on chronic prednisolone sodium phosphate oral solution therapy who develop systemic fungal infections, prednisolone sodium phosphate oral solution withdrawal or dosage reduction is recommended.
Amebiasis: Corticosteroids, including prednisolone sodium phosphate oral solution, may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating prednisolone sodium phosphate oral solution in patients who have spent time in the tropics or patients with unexplained diarrhea.
Strongyloides Infestation: Corticosteroids, including prednisolone sodium phosphate oral solution, should be used with great care in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Cerebral Malaria: Avoid corticosteroids, including prednisolone sodium phosphate oral solution, in patients with cerebral malaria.
Ophthalmic: Use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to bacteria, fungi or viruses. The use of oral corticosteroids is not recommended in the treatment of optic neuritis and may lead to an increase in the risk of new episodes. Corticosteroids should not be used in active ocular herpes simplex.
Kaposi’s Sarcoma: Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement of Kaposi’s sarcoma.
Vaccination: Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. Killed or inactivated vaccines may be administered, however, the response to such vaccines cannot be predicted. Immunization procedures may be undertaken in patients who are receiving corticosteroids as replacement therapy, e.g., for Addison’s disease.
Cardio-renal: Average and large doses of hydrocortisone or cortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Endocrine: Corticosteroids can produce reversible hypothalamic-pituitary adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment.
Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid status of the patient may necessitate adjustment in dosage.
-
PRECAUTIONS
General: The lowest possible dose of corticosteroid should be used to control the condition under treatment, and when reduction in dosage is possible, the reduction should be gradual.
Since complications of treatment with glucocorticoids are dependent on the size of the dose and the duration of treatment, a risk/benefit decision must be made in each individual case as to dose and duration of treatment and as to whether daily or intermittent therapy should be used.
There is an enhanced effect of corticosteroids in patients with hypothyroidism and in those with cirrhosis.
Cardio-renal: As sodium retention with resultant edema and potassium loss may occur in patients receiving corticosteroids, these agents should be used with caution in patients with hypertension, congestive heart failure, or renal insufficiency.
Endocrine: Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.
Gastrointestinal: Steroids should be used with caution in nonspecific ulcerative colitis, if there is a probability of impending perforation, abscess or other pyogenic infection; diverticulitis; fresh intestinal anastomoses; active or latent peptic ulcer.
Signs of peritoneal irritation following gastrointestinal perforation in patients receiving corticosteroids may be minimal or absent.
Musculoskeletal: Corticosteroids decrease bone formation and increase bone resorption both through their effect on calcium regulation (i.e., decreasing absorption and increasing excretion) and inhibition of osteoblast function. This, together with a decrease in the protein matrix of the bone secondary to an increase in protein catabolism, and reduced sex hormone production, may lead to inhibition of bone growth in children and adolescents and the development of osteoporosis at any age. Special consideration should be given to patients at increased risk of osteoporosis (i.e., post-menopausal women) before initiating corticosteroid therapy.
Neuro-psychiatric: Although controlled clinical trials have shown corticosteroids to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that they affect the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of corticosteroids are necessary to demonstrate a significant effect. (See DOSAGE AND ADMINISTRATION.)
An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (e.g., myasthenia gravis), or in patients receiving concomitant therapy with neuromuscular blocking drugs (e.g., pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevation of creatinine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Ophthalmic: Intraocular pressure may become elevated in some individuals. If steroid therapy is continued for more than 6 weeks, intraocular pressure should be monitored.
Information for Patients: Patients should be warned not to discontinue the use of prednisolone sodium phosphate oral solution abruptly or without medical supervision, to advise any medical attendants that they are taking it, and to seek medical advice at once should they develop fever or other signs of infection.
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chicken pox or measles. Patients should also be advised that if they are exposed, medical advice should be sought without delay.
Drug Interactions: Drugs such as barbiturates, phenytoin, ephedrine, and rifampin, which induce hepatic microsomal drug metabolizing enzyme activity may enhance metabolism of prednisolone and require that the dosage of prednisolone sodium phosphate oral solution be increased.
Increased activity of both cyclosporin and corticosteroids may occur when the two are used concurrently. Convulsions have been reported with this concurrent use.
Estrogens may decrease the hepatic metabolism of certain corticosteroids thereby increasing their effect.
Ketoconazole has been reported to decrease the metabolism of certain corticosteroids by up to 60% leading to an increased risk of corticosteroid side effects.
Coadministration of corticosteroids and warfarin usually results in inhibition of response to warfarin, although there have been some conflicting reports. Therefore, coagulation indices should be monitored frequently to maintain the desired anticoagulant effect.
Concomitant use of aspirin (or other non-steroidal anti-inflammatory agents) and corticosteroids increases the risk of gastrointestinal side effects. Aspirin should be used cautiously in conjunction with corticosteroids in hypoprothrombinemia. The clearance of salicylates may be increased with concurrent use of corticosteroids.
When corticosteroids are administered concomitantly with potassium-depleting agents (i.e., diuretics, amphotericin-B), patients should be observed closely for development of hypokalemia. Patients on digitalis glycosides may be at increased risk of arrhythmias due to hypokalemia.
Concomitant use of anticholinesterase agents and corticosteroids may produce severe weakness in patients with myasthenia gravis. If possible, anticholinesterase agents should be withdrawn at least 24 hours before initiating corticosteroid therapy.
Due to inhibition of antibody response, patients on prolonged corticosteroid therapy may exhibit a diminished response to toxoids and live or inactivated vaccines. Corticosteroids may also potentiate the replication of some organisms contained in live attenuated vaccines. If possible, routine administration of vaccines or toxoids should be deferred until corticosteroid therapy is discontinued.
Because corticosteroids may increase blood glucose concentrations, dosage adjustments of antidiabetic agents may be required.
Corticosteroids may suppress reactions to skin tests.
Pregnancy: Teratogenic Effects:
Pregnancy Category C.
Prednisolone has been shown to be teratogenic in many species when given in doses equivalent to the human dose. Animal studies in which prednisolone has been given to pregnant mice, rats, and rabbits have yielded an increased incidence of cleft palate in the offspring. There are no adequate and well-controlled studies in pregnant women. Prednisolone sodium phosphate oral solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born to mothers who have received corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
Nursing Mothers: Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution should be exercised when prednisolone sodium phosphate oral solution is administered to a nursing woman.
Pediatric Use: The efficacy and safety of prednisolone in the pediatric population are based on the well-established course of effect of corticosteroids which is similar in pediatric and adult populations. Published studies provide evidence of efficacy and safety in pediatric patients for the treatment of nephrotic syndrome (>2 years of age), and aggressive lymphomas and leukemias (>1 month of age). However, some of these conclusions and other indications for pediatric use of corticosteroid, e.g., severe asthma and wheezing, are based on adequate and well-controlled trials conducted in adults, on the premises that the course of the diseases and their pathophysiology are considered to be substantially similar in both populations.
The adverse effects of prednisolone in pediatric patients are similar to those in adults (see ADVERSE REACTIONS). Like adults, pediatric patients should be carefully observed with frequent measurements of blood pressure, weight, height, intraocular pressure, and clinical evaluation for the presence of infection, psychosocial disturbances, thromboembolism, peptic ulcers, cataracts, and osteoporosis. Children who are treated with corticosteroids by any route, including systemically administered corticosteroids, may experience a decrease in their growth velocity. This negative impact of corticosteroids on growth has been observed at low systemic doses and in the absence of laboratory evidence of HPA axis suppression (i.e., cosyntropin stimulation and basal cortisol plasma levels). Growth velocity may therefore be a more sensitive indicator of systemic corticosteroid exposure in children than some commonly used tests of HPA axis function. The linear growth of children treated with corticosteroids by any route should be monitored, and the potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of other treatment alternatives. In order to minimize the potential growth effects of corticosteroids, children should be titrated to the lowest effective dose.
Geriatric Use: Clinical studies of prednisolone sodium phosphate oral solution did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience with prednisolone sodium phosphate has not identified differences in responses between the elderly and younger patients. However, the incidence of corticosteroid-induced side effects may be increased in geriatric patients and appear to be dose-related.
Osteoporosis is the most frequently encountered complication, which occurs at a higher incidence rate in corticosteroid-treated geriatric patients as compared to younger populations and in age-matched controls. Losses of bone mineral density appear to be greatest early on in the course of treatment and may recover over time after steroid withdrawal or use of lower doses (i.e., £5 mg/day). Prednisolone doses of 7.5 mg/day or higher have been associated with an increased relative risk of both vertebral and nonvertebral fractures, even in the presence of higher bone density compared to patients with involutional osteoporosis.
Routine screening of geriatric patients, including regular assessments of bone mineral density and institution of fracture prevention strategies along with regular review of prednisolone sodium phosphate indication should be undertaken to minimize complications and keep the dose at the lowest acceptable level. Co-administration of bisphosphonates has been shown to retard the rate of bone loss in corticosteroid-treated males and postmenopausal females, and these agents are recommended in the prevention and treatment of corticosteroid-induced osteoporosis.
It has been reported that equivalent weight-based doses yield higher total and unbound prednisolone plasma concentrations and reduced renal and non-renal clearance in elderly patients compared to younger populations. However, it is not clear whether dosing reductions would be necessary in elderly patients, since these pharmacokinetic alterations may be offset by age-related differences in responsiveness of target organs and/or less pronounced suppression of adrenal release of cortisol. Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of communicant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see CLINICAL PHARMACOLOGY).
Teratogenic Effects
Prednisolone has been shown to be teratogenic in many species when given in doses equivalent to the human dose. Animal studies in which prednisolone has been given to pregnant mice, rats, and rabbits have yielded an increased incidence of cleft palate in the offspring. There are no adequate and wellcontrolled studies in pregnant women. Prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born to mothers who have received corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution should be exercised when prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) is administered to a nursing woman.
Pediatric Use
The efficacy and safety of prednisolone in the pediatric population are based on the well-established course of effect of corticosteroids which is similar in pediatric and adult populations. Published studies provide evidence of efficacy and safety in pediatric patients for the treatment of nephrotic syndrome (>2 years of age), and aggressive lymphomas and leukemias (>1 month of age). However, some of these conclusions and other indications for pediatric use of corticosteroid, e.g., severe asthma and wheezing, are based on adequate and well-controlled trials conducted in adults, on the premises that the course of the diseases and their pathophysiology are considered to be substantially similar in both populations.
The adverse effects of prednisolone in pediatric patients are similar to those in adults (see ADVERSE REACTIONS). Like adults, pediatric patients should be carefully observed with frequent measurements of blood pressure, weight, height, intraocular pressure, and clinical evaluation for the presence of infection, psychosocial disturbances, thromboembolism, peptic ulcers, cataracts, and osteoporosis. Children who are treated with corticosteroids by any route, including systemically administered corticosteroids, may experience a decrease in their growth velocity. This negative impact of corticosteroids on growth has been observed at low systemic doses and in the absence of laboratory evidence of HPA axis suppression (i.e., cosyntropin stimulation and basal cortisol plasma levels). Growth velocity may therefore be a more sensitive indicator of systemic corticosteroid exposure in children than some commonly used tests of HPA axis function. The linear growth of children treated with corticosteroids by any route should be monitored, and the potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of other treatment alternatives. In order to minimize the potential growth effects of corticosteroids, children should be titrated to the lowest effective dose.
Geriatric Use
Clinical studies of prednisolone sodium phosphate oral solution did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience with prednisolone sodium phosphate has not identified differences in responses between the elderly and younger patients. However, the incidence of corticosteroid-induced side effects may be increased in geriatric patients and appear to be dose-related. Osteoporosis is the most frequently encountered complication, which occurs at a higher incidence rate in corticosteroid-treated geriatric patients as compared to younger populations and in age-matched controls. Losses of bone mineral density appear to be greatest early on in the course of treatment and may recover over time after steroid withdrawal or use of lower doses (i.e., ≤5 mg/day). Prednisolone doses of 7.5 mg/day or higher have been associated with an increased relative risk of both vertebral and nonvertebral fractures, even in the presence of higher bone density compared to patients with involutional osteoporosis.
Routine screening of geriatric patients, including regular assessments of bone mineral density and institution of fracture prevention strategies along with regular review of prednisolone sodium phosphate indication should be undertaken to minimize complications and keep the dose at the lowest acceptable level. Co-administration of bisphosphonates has been shown to retard the rate of bone loss in corticosteroidtreated males and postmenopausal females, and these agents are recommended in the prevention and treatment of corticosteroid-induced osteoporosis.
It has been reported that equivalent weight-based doses yield higher total and unbound prednisolone plasma concentrations and reduced renal and non-renal clearance in elderly patients compared to younger populations. However, it is not clear whether dosing reductions would be necessary in elderly patients, since these pharmacokinetic alterations may be offset by age-related differences in responsiveness of target organs and/ or less pronounced suppression of adrenal release of cortisol. Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of communicant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see CLINICAL PHARMACOLOGY).
-
ADVERSE REACTIONS (listed alphabetically under each subsection)
Cardiovascular: Hypertrophic cardiomyopathy in premature infants.
Dermatologic: Facial erythema; increased sweating; impaired wound healing; may suppress reactions to skin tests; petechiae and ecchymoses; thin fragile skin; urticaria; edema.
Endocrine: Decreased carbohydrate tolerance; development of cushingoid state; hirsutism; increased requirements for insulin or oral hypoglycemic agents in diabetic patients; manifestations of latent diabetes mellitus; menstrual irregularities; secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness; suppression of growth in children.
Fluid and Electrolyte Disturbances: Congestive heart failure in susceptible patients; fluid retention; hypertension; hypokalemic alkalosis; potassium loss; sodium retention.
Gastrointestinal: Abdominal distention; elevation in serum liver enzyme levels (usually reversible upon discontinuation); pancreatitis; peptic ulcer with possible perforation and hemorrhage; ulcerative esophagitis.
Metabolic: Negative nitrogen balance due to protein catabolism.
Musculoskeletal: Aseptic necrosis of femoral and humeral heads; loss of muscle mass; muscle weakness; osteoporosis; pathologic fracture of long bones; steroid myopathy; tendon rupture; vertebral compression fractures.
Neurological: Convulsions; headache; increased intracranial pressure with papilledema (pseudotumor cerebri) usually following discontinuation of treatment; psychic disorders; vertigo.
Ophthalmic: Exophthalmos; glaucoma; increased intraocular pressure; posterior subcapsular cataracts.
Other: Increased appetite; malaise; nausea; weight gain.
-
OVERDOSAGE
The effects of accidental ingestion of large quantities of prednisolone over a very short period of time have not been reported, but prolonged use of the drug can produce mental symptoms, moon face, abnormal fat deposits, fluid retention, excessive appetite, weight gain, hypertrichosis, acne, striae, ecchymosis, increased sweating, pigmentation, dry scaly skin, thinning scalp hair, increased blood pressure, tachycardia, thrombophlebitis, decreased resistance to infection, negative nitrogen balance with delayed bone and wound healing, headache, weakness, menstrual disorders, accentuated menopausal symptoms, neuropathy, fractures, osteoporosis, peptic ulcer, decreased glucose tolerance, hypokalemia, and adrenal insufficiency. Hepatomegaly and abdominal distention have been observed in children.
Treatment of acute overdosage is by immediate gastric lavage or emesis followed by supportive and symptomatic therapy. For chronic overdosage in the face of severe disease requiring continuous steroid therapy, the dosage of prednisolone may be reduced only temporarily, or alternate day treatment may be introduced.
CLOSE
-
DOSAGE AND ADMINISTRATION
The initial dosage of prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) may vary from 1 mL to 12 mL (5 to 60 mg prednisolone base) per day depending on the specific disease entity being treated. In situations of less severity, lower doses will generally suffice while in selected patients higher initial doses may be required. The initial dosage should be maintained or adjusted until a satisfactory response is noted. If after a reasonable period of time, there is a lack of satisfactory clinical response, prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) should be discontinued and the patient placed on other appropriate therapy. IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT. After a favorable response is noted, the proper maintenance dosage should be determined by decreasing the initial drug dosage in small decrements at appropriate time intervals until the lowest dosage which will maintain an adequate clinical response is reached. It should be kept in mind that constant monitoring is needed in regard to drug dosage. Included in the situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient’s individual drug responsiveness, and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment; in this latter situation it may be necessary to increase the dosage of prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) for a period of time consistent with the patient’s condition. If after long term therapy the drug is to be stopped, it is recommended that it be withdrawn gradually rather than abruptly.
In the treatment of acute exacerbations of multiple sclerosis, daily doses of 200 mg of prednisolone for a week followed by 80 mg every other day or 4 to 8 mg dexamethasone every other day for one month have been shown to be effective.
In pediatric patients, the initial dose of prednisolone sodium phosphate oral solution (25 mg prednisolone per 5 mL) may vary depending on the specific disease entity being treated. The range of initial doses is 0.14 to 2 mg/kg/day in three or four divided doses (4 to 60 mg/m2bsa/day).
The standard regimen used to treat nephrotic syndrome in pediatric patients is 60 mg/m2/day given in three divided doses for 4 weeks, followed by 4 weeks of single dose alternate-day therapy at 40 mg/m2/day.
The National Heart, Lung, and Blood Institute (NHLBI) recommended dosing for systemic prednisone, prednisolone or methylprednisolone in children whose asthma is uncontrolled by inhaled corticosteroids and long-acting bronchodilators is 1-2 mg/kg/day in single or divided doses. It is further recommended that short course, or “burst” therapy, be continued until a child achieves a peak expiratory flow rate of 80% of his or her personal best or symptoms resolve. This usually requires 3 to 10 days of treatment, although it can take longer. There is no evidence that tapering the dose after improvement will prevent a relapse.
For the purpose of comparison, 5 mL of prednisolone sodium phosphate oral solution (33.6 mg prednisolone sodium phosphate) is equivalent to the following milligram dosage of the various glucocorticoids:
Cortisone, 125 Triamcinolone, 20
Hydrocortisone, 100 Paramethasone, 10
Prednisolone, 25 Betamethasone, 3.75
Prednisone, 25 Dexamethasone, 3.75
Methylprednisolone, 20These dose relationships apply only to oral or intravenous administration of these compounds. When these substances or their derivatives are injected intramuscularly or into joint spaces, their relative properties may be greatly altered.
-
HOW SUPPLIED
ach 5 mL (teaspoonful) of Prednisolone Sodium Phosphate Oral Solution contains 33.6 mg prednisolone sodium phosphate (25 mg prednisolone base) in a pale yellow, grape flavored solution.
NDC 0178-0582-08 8 fl oz (237 mL) bottle
Dispense in tight, light-resistant glass or PET plastic containers as defined in the USP.
Store refrigerated, 2° to 8°C (36° to 46°F).
Professional sample:
NDC 0178-0582-01 1 fl oz (30 mL) sample bottle
Dispense in tight, light-resistant glass or PET plastic containers as defined in the USP.
Store at 20° to 25°C (68° to 77°F).
Keep tightly closed and out of the reach of children.
Manufactured for:
BioComp Pharma, Inc.
San Antonio, TX 78230 1355Each 5 mL (teaspoonful) of Prednisolone Sodium Phosphate Oral Solution
contains 33.6 mg prednisolone sodium phosphate (25 mg prednisolone
base) in a pale yellow, grape flavored solution.
NDC 44523-182-08 8 fl oz (237 mL) bottle
Dispense in tight, light-resistant glass or PET plastic containers as defined in
the USP.
Store refrigerated, 2° to 8°C (36° to 46°F).
Keep tightly closed and out of the reach of children. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREDNISOLONE SODIUM PHOSPHATE ORAL SOLUTION
prednisolone sodium phosphate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:44523-182 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PREDNISOLONE SODIUM PHOSPHATE (UNII: IV021NXA9J) (PREDNISOLONE - UNII:9PHQ9Y1OLM) PREDNISOLONE 25 mg in 5 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44523-182-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091396 10/28/2019 Labeler - BioComp Pharma, Inc. (829249718) Registrant - BioComp Pharma, Inc. (829249718) Establishment Name Address ID/FEI Business Operations PAI Holdings, LLC dba Pharmaceutical Associates, Inc. and dba PAI Pharma 097630693 analysis(44523-182) , pack(44523-182) , manufacture(44523-182) , label(44523-182)