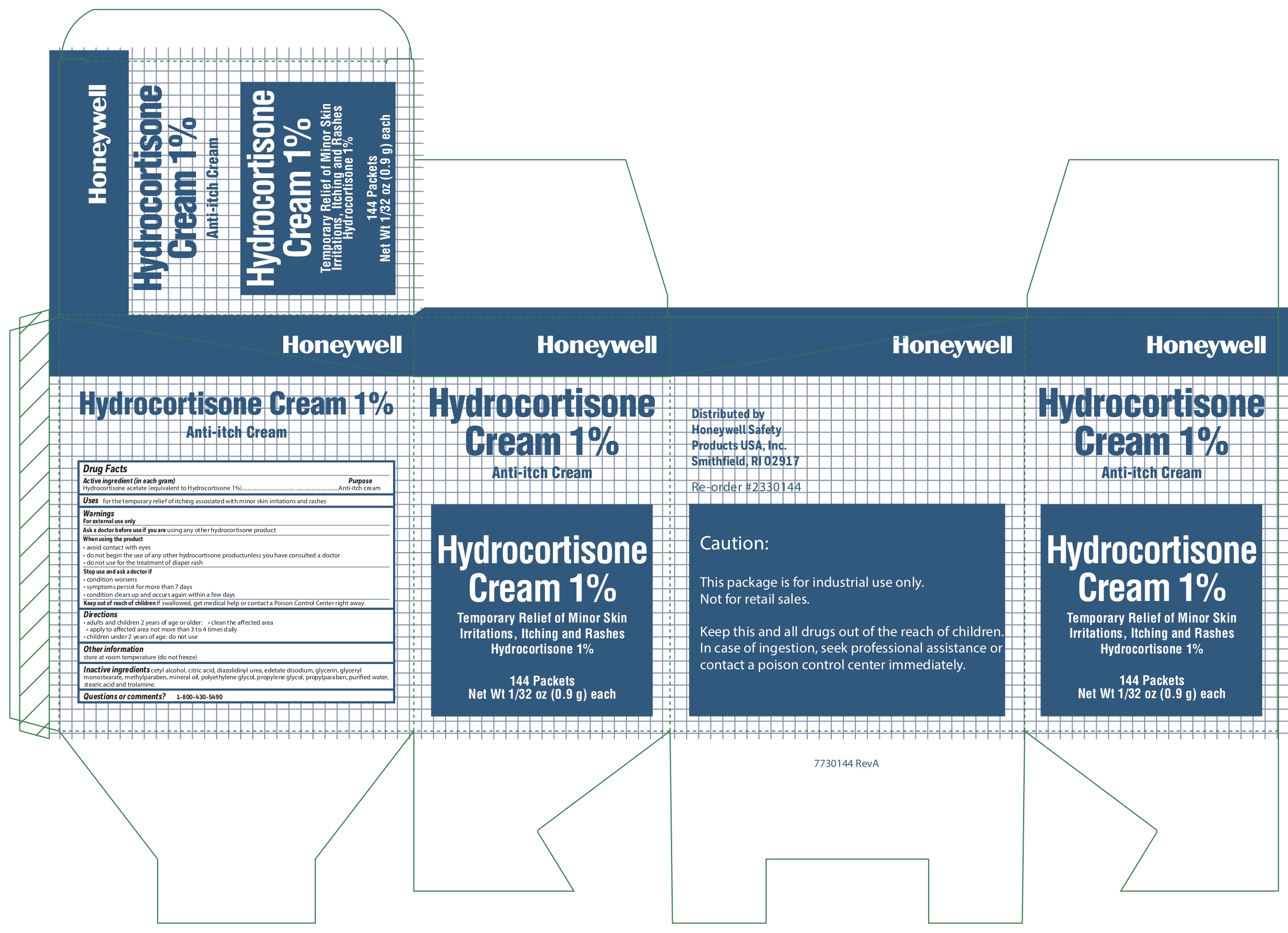
Label: HYDROCORTISONE- anti-itch cream
-
NDC Code(s):
0498-0801-01,
0498-0801-02,
0498-0801-03,
0498-0801-32, view more0498-0801-33, 0498-0801-34, 0498-0801-35
- Packager: Honeywell Safety Products USA, inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Ask a doctor before use if
Ask a doctor before use if you are using any other hydrocortisone product
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE
anti-itch creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-0801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CETYL ALCOHOL (UNII: 936JST6JCN) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0801-03 25 in 1 BOX, UNIT-DOSE 10/15/2019 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC:0498-0801-02 144 in 1 BOX, UNIT-DOSE 10/15/2019 2 0.9 g in 1 PACKET; Type 0: Not a Combination Product 3 NDC:0498-0801-01 1728 in 1 CARTON 10/15/2019 3 0.9 g in 1 PACKET; Type 0: Not a Combination Product 4 NDC:0498-0801-32 20 in 1 BOX, UNIT-DOSE 10/15/2019 4 0.9 g in 1 PACKET; Type 0: Not a Combination Product 5 NDC:0498-0801-33 100 in 1 BOX, UNIT-DOSE 10/15/2019 5 0.9 g in 1 PACKET; Type 0: Not a Combination Product 6 NDC:0498-0801-34 10 in 1 BOX, UNIT-DOSE 10/15/2019 6 0.9 g in 1 PACKET; Type 0: Not a Combination Product 7 NDC:0498-0801-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product 10/15/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/15/2019 Labeler - Honeywell Safety Products USA, inc (118768815) Registrant - Honeywell Safety Products USA, Inc (118768815)