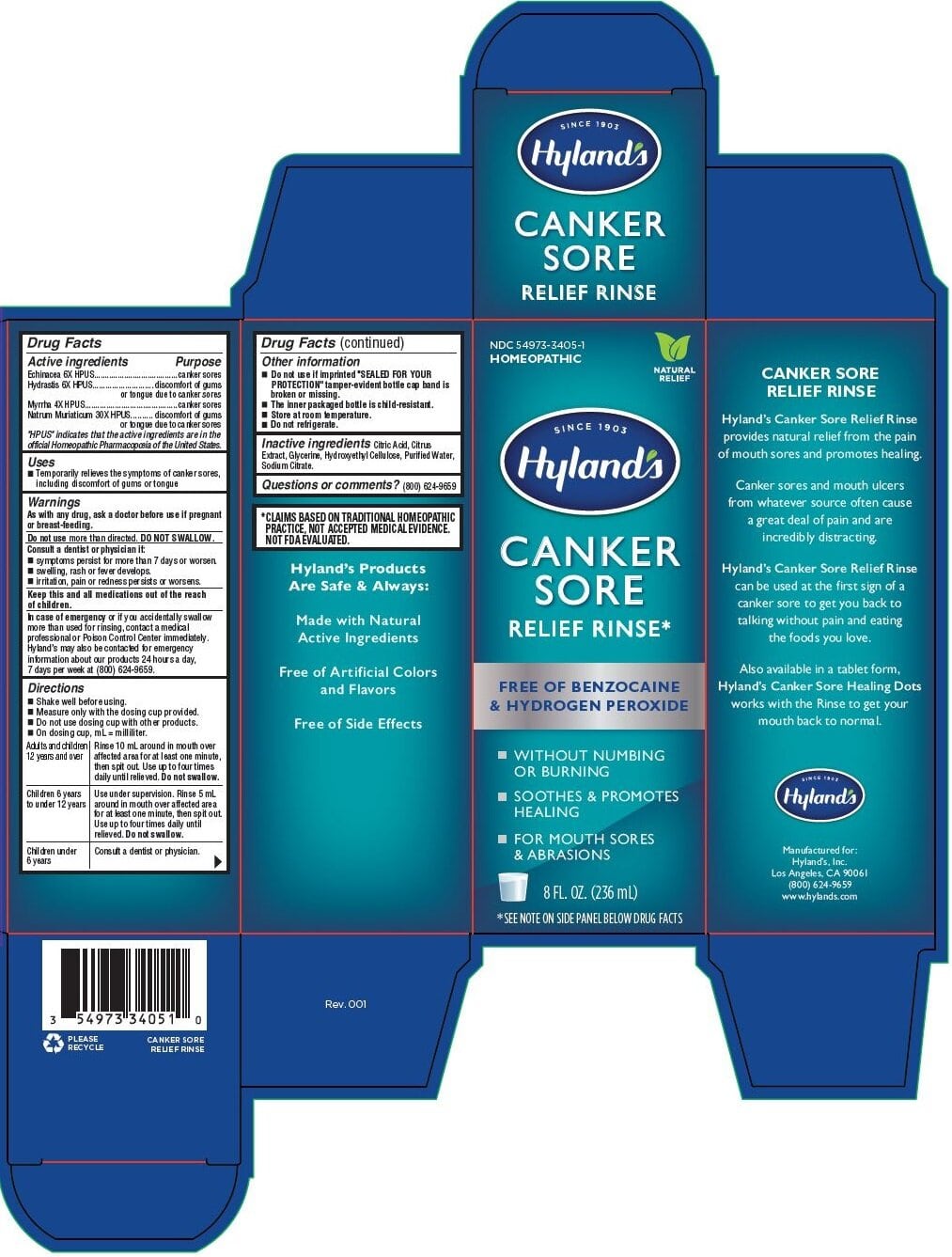
CANKER SORE RELIEF RINSE- goldenseal,myrrh,echinacea angustifolia and sodium chloride liquid
Hyland's
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Hyland's Canker Sore Relief Rinse
Active ingredients
Echinacea 6X HPUS
Hydrastis 6X HPUS
Myrrha 4X HPUS
Natrum Muriaticum 30X HPUS
"HPUS" indicates that the active ingredients are in the
official Homeopathic Pharmacopoeia of the United States.
Warnings
As with any drug, ask a doctor before use if pregnant
or breast-feeding.
Directions
- Shake well before using
- Measure only with the dosing cup provided.
- Do not use dosing cup with other products.
- On dosing cup, mL = milliliter.
|
Adults and children 12 years and over |
Rinse 10 mL around in mouth over affected area for at least one minute, then spit out. Use up to four times daily until relieved. Do not swallow. |
|
Children 6 years to under 12 years |
Use under supervision. Rinse 5 mL around in mouth over affected area for at least one minute, then spit out. Use up to four times daily until relieved. Do not swallow. |
|
Children under 6 years |
Consult a dentist or physician. |
Drug Facts (continued)
Other information
- Do not use if imprinted "SEALED FOR YOUR
- PROTECTION" tamper-evident bottle cap band is broken or missing.
- The inner packaged bottle is child-resistant.
- Store at room temperature.
- Do not refrigerate.
* CLAIMS BASED ON TRADITIONAL HOMEOPATHIC
PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE.
NOT FDA EVALUATED.
PRINCIPAL DISPLAY PANEL - 236 mL BOTTLE CARTON
NDC 54973-3405-1
HOMEOPATHIC
NATURAL
RELIEF
SINCE 1903
Hyland's
CANKER
SORE
RELIEF RINSE*
FREE OF BENZOCAINE
& HYDROGEN PEROXIDE
• WITHOUT NUMBING
OR BURNING
• SOOTHES & PROMOTES
HEALING
• FOR MOUTH SORES
& ABRASIONS
8 FL. OZ. (236 mL)
*SEE NOTE ON SIDE PANEL BELOW DRUG FACTS
| CANKER SORE RELIEF RINSE
goldenseal,myrrh,echinacea angustifolia and sodium chloride liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Hyland's (028570695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Applied Laboratories, Inc. | 876754375 | manufacture(54973-3405) , pack(54973-3405) , label(54973-3405) | |