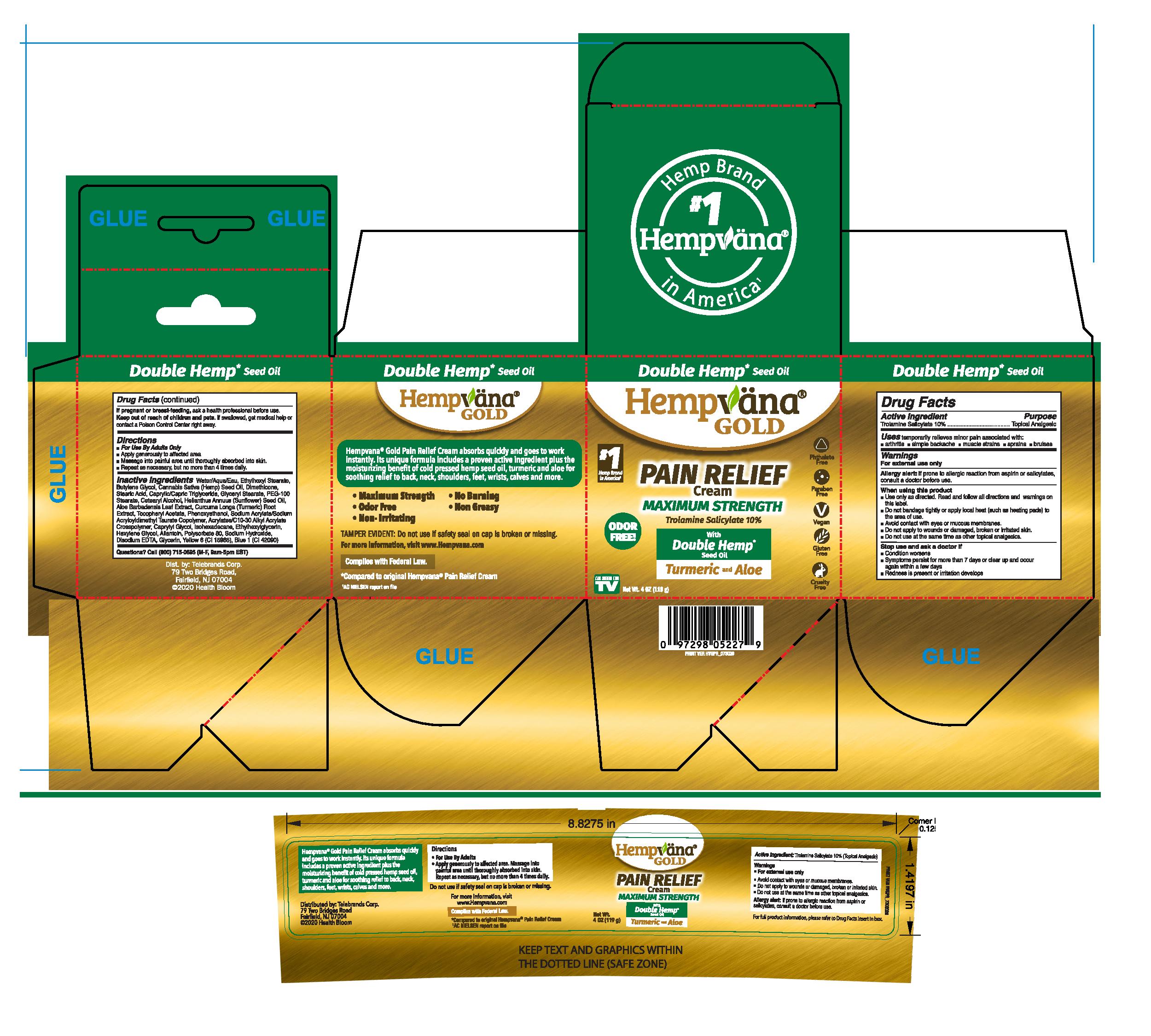
HEMPVANA GOLD PAIN RELIEF- trolamine salicylate cream
Telebrands Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hempvana Pain Relief Cream
Uses
temporarily relieves minor pain associated with:
• arthritis • simple backache • muscle strains • sprains • bruises
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- Use only as directed. Read and follow all directions and warnings on this label.
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use.
- Avoid contact with eyes or mucous membranes.
- Do not apply to wounds or damaged, broken or irritated skin.
- Do not use at the same time as other topical analgesics.
Directions
- For Use By Adults Only
- Apply generously to affected area.
- Massage into painful area until thoroughly absorbed into skin.
- Repeat as necessary, but no more than 4 times daily.
Inactive Ingredients
Water/Aqua, Ethylhexyl Stearate, Butylene Glycol, Cannabis Sativa (Hemp) Seed Oil, Dimethicone, Stearic Acid, Caprylic/Capric Triglyceride, Glyceryl Stearate, PEG-100 Stearate, Cetearyl Alcohol, Helianthus Annuus (Sunflower) Seed Oil, Aloe Barbadensis Leaf Extract, Curcuma Longa (Turmeric) Root Extract, Tocopheryl Acetate, Phenoxyethanol, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylyl Glycol, Isohexadecane, Ethylhexylglycerin, Hexylene Glycol, Allantoin, Polysorbate 80, Sodium Hydroxide, Disodium EDTA, Glycerin, Yellow 6 (CI 15985), Blue 1 (CI 42090)
Hempvana ® Pain Relief Cream absorbs quickly and goes to work instantly. Its unique formula includes a proven active ingredient plus the moisturizing benefit of cold pressed hemp seed* extract for soothing relief to back, neck, shoulders, feet, wrists, calves and more.
- Maximum Strength
- Odor Free
- Non-Irritating
- No Burning
- Non Greasy
TAMPER EVIDENT: Do not use if safety seal on cap is broken or missing.
For more information, visit www.HempvanaStore.com
*Complies with Federal Law. Hemp seed extract in the form of oil made by cold-pressing industrial hemp seeds.
cultivar.
**Compared to original Hempvana Pain Relief Cream
Dist. by: Telebrands Corp.
79 Two Bridges Road,
Fairfield, NJ 07004
| HEMPVANA GOLD
PAIN RELIEF
trolamine salicylate cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Telebrands Corp (177266558) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Neutraderm, Inc. | 146224444 | manufacture(73287-003) | |