RECTICARE ADVANCED- glycerin and lidocaine and phenylephrine hcl cloth
Ferndale Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
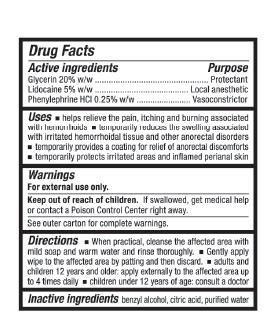
RectiCare Advanced Hemorrhoidal Wipes
Purpose
Glycerin 20%w/w.........................................Protectant
Lidocaine 5% w/w........................................Local Aneshtetic
Phenylephrine HCl 0.25% w/w.....................Vasoconstrictor
Uses
- helps relieve the pain, itching and burning associated with hemorrhoids
- temporarily reduces the swelling associated with irritated hemorrhoidal tissue and other anorectal disorders
- temporarily provides a coating for relief of anorectal discomforts
- temporarily protects irritated areas and inflamed perianal skin
Warnings
For external use only.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thryoid disease
- diabetes
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug for high blood pressure or depression
When using this product
- avoid contact with eyes
- do not exceed recommended daily dosage unless directed by a doctor
- do not put this product into the rectum by using fingers or any mechanical device or applicator
Directions
- When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly.
- Gently apply wipe to the affected area by patting and then discard.
- adults and children 12 years and older: apply externally to the affected area up to 4 times daily
- children under 12 years of age: consult a doctor
| RECTICARE ADVANCED
glycerin and lidocaine and phenylephrine hcl cloth |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Ferndale Laboratories, Inc. (005320536) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ferndale Laboratories, Inc. | 005320536 | manufacture(0496-0979) | |
Revised: 11/2019
Document Id: 97a36b26-c730-7afc-e053-2995a90a0ea4
Set id: 94d07482-60b0-4a45-8f79-9723de3cf1ba
Version: 2
Effective Time: 20191118
Ferndale Laboratories, Inc.