TILIA EUROPAEA- tilia x europaea flower pellet
Washington Homeopathic Products
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Tilia europaea X
STOP USE AND ASK DOCTOR
If symptoms persist/worsen or if pregnant/nursing, stop use and consult your practitioner.
DIRECTIONS
Adults: Dissolve 3 to 5 under the tongue three times a day or as directed by Lic. Practitioner. Take at greater intervals as condition subsides. Children: Dissolve 3 to 5 under the tongue three times a day or as directed by Lic. Practitioner. Take at greater intervals as condition subsides.
PRINCIPAL DISPLAY PANEL
Enter section text The OTC potency range of TILIA EUROPAEA is 2x–30x, 1c–30c, 200c, 1m, 10m, 50m, and CM.
Availability is subject to change.
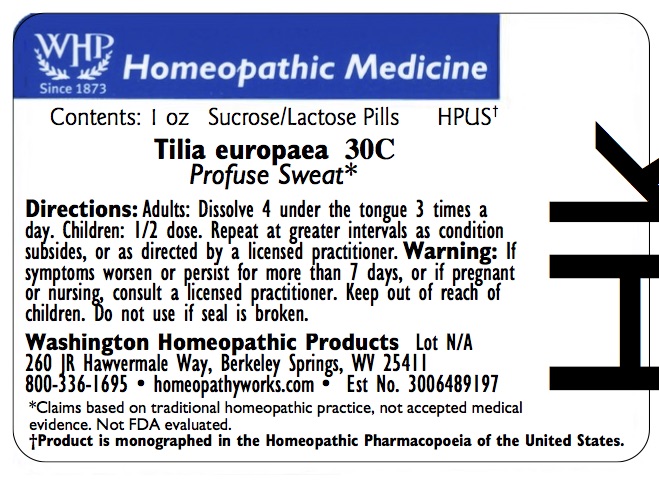
All WHP single remedies are made to order; thus, the labels are printed on the same label stock as the orders are filled.
‘Bottle Size’ and ‘Potency’ vary on the label depending on customer choice.
Standard bottle sizes for pellet-form remedies are 2 dram, 4 dram, 1 ounce, 2 ounce, and 4 ounce.
here
| TILIA EUROPAEA
tilia x europaea flower pellet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Washington Homeopathic Products (084929389) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Washington Homeopathic Products | 084929389 | manufacture(68428-680) | |