COLGATE TOTAL DAILY REPAIR- sodium fluoride and triclosan paste, dentifrice
Colgate-Palmolive Company
----------
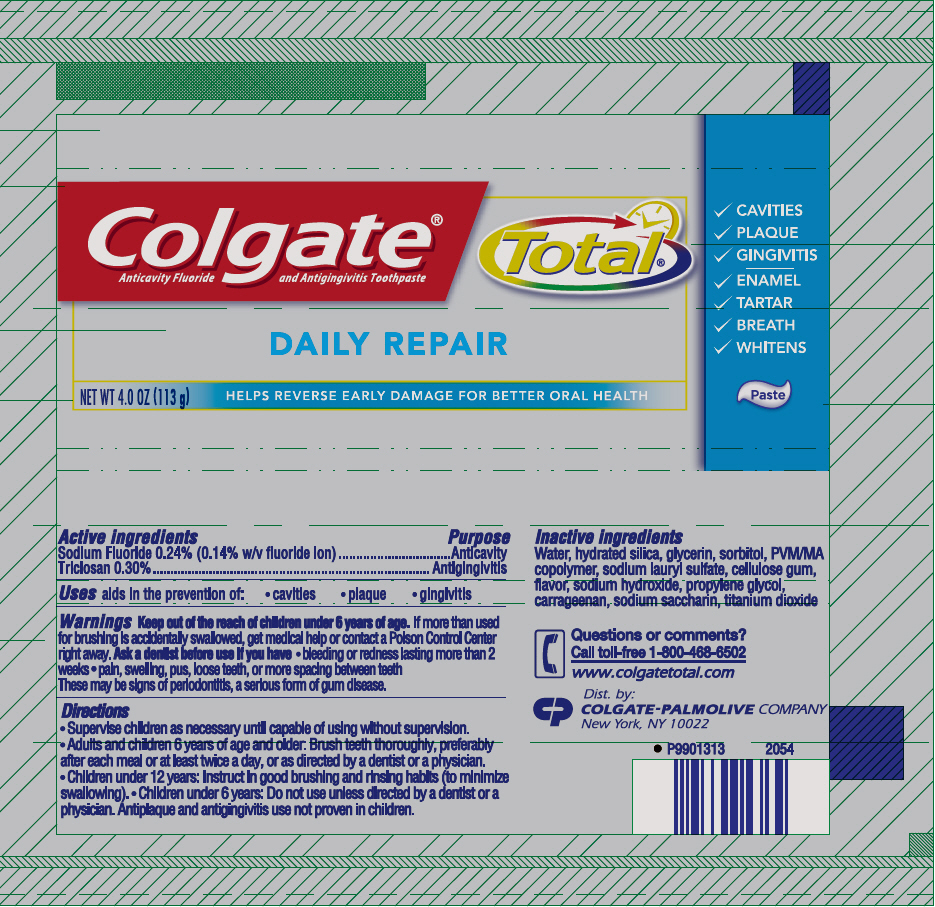
Colgate® Total® Daily Repair Paste
Warnings
Keep out of the reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Ask a dentist before use if you have
- bleeding or redness lasting more than 2 weeks
- pain, swelling, pus, loose teeth, or more spacing between teeth
These may be signs of periodontitis, a serious form of gum disease.
Directions
- Supervise children as necessary until capable of using without supervision.
- Adults and children 6 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or a physician.
- Children under 12 years: Instruct in good brushing and rinsing habits (to minimize swallowing).
- Children under 6 years: Do not use unless directed by a dentist or a physician. Antiplaque and antigingivitis use not proven in children.
| COLGATE TOTAL DAILY REPAIR
sodium fluoride and triclosan paste, dentifrice |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Colgate-Palmolive Company (001344381) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Colgate-Palmolive | 785047999 | MANUFACTURE(35000-168) | |
Revised: 12/2019
Document Id: d51ecb55-a8a1-4822-bee1-bd616a7484af
Set id: 933a6e9b-06a6-4fca-bd28-40362cfbec90
Version: 9
Effective Time: 20191209
Colgate-Palmolive Company