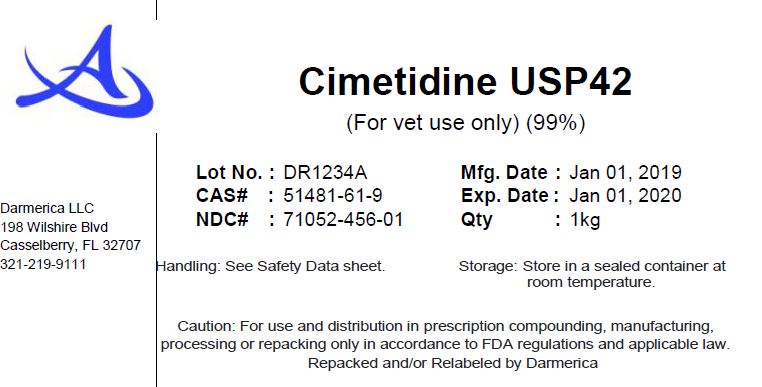
CIMETIDINE- cimetidine powder
DARMERICA, LLC
----------
Cimetidine
| CIMETIDINE
cimetidine powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - DARMERICA, LLC (080233052) |
Revised: 7/2020
Document Id: ab2100e6-da06-b386-e053-2995a90ab118
Set id: 92392ac2-d3c8-6161-e053-2a95a90a4b81
Version: 4
Effective Time: 20200723
DARMERICA, LLC