TENHEALER PAIN RUB- camphor (synthetic), menthol, methyl salicylate lotion
BIO HEALING THERAPY INDUSTRIES INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
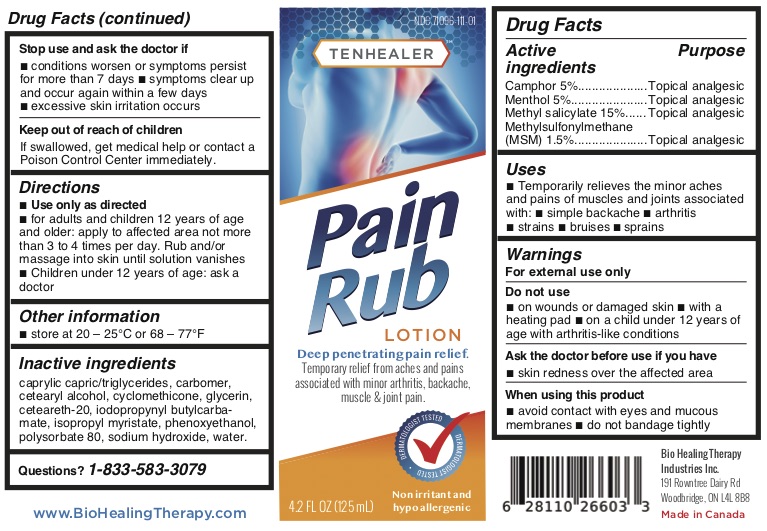
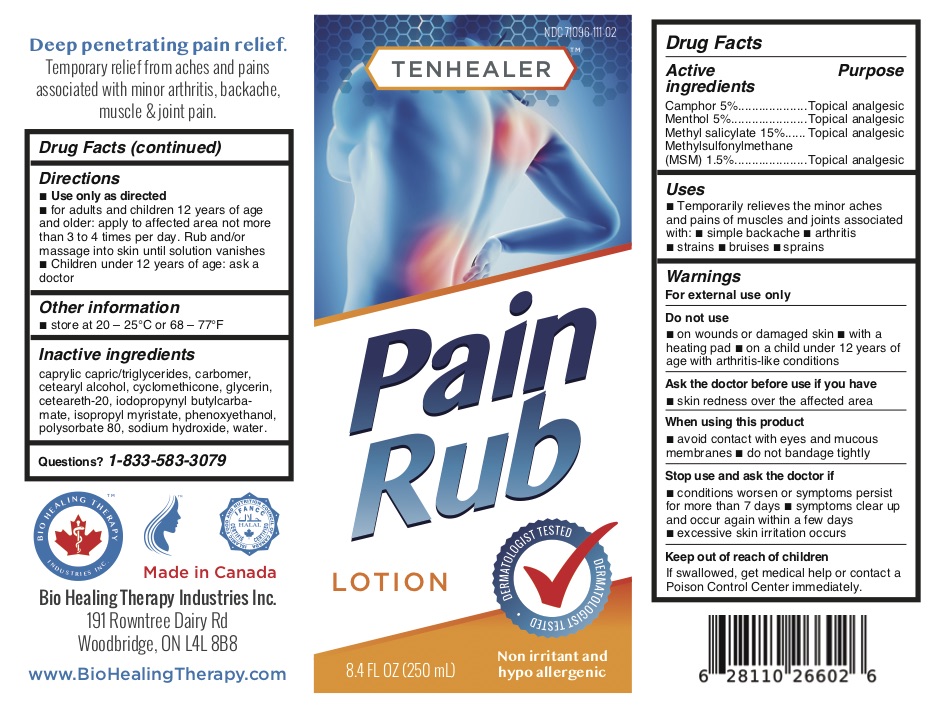
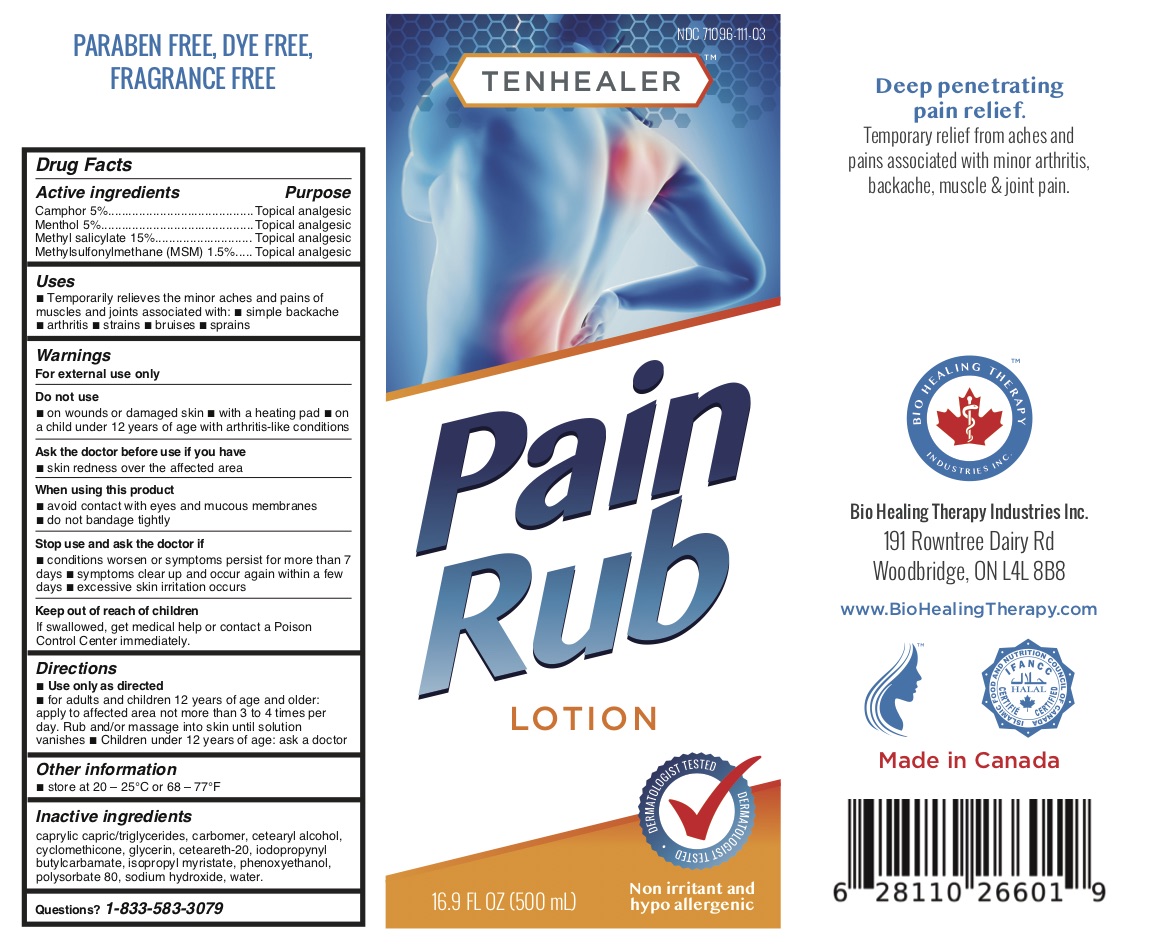
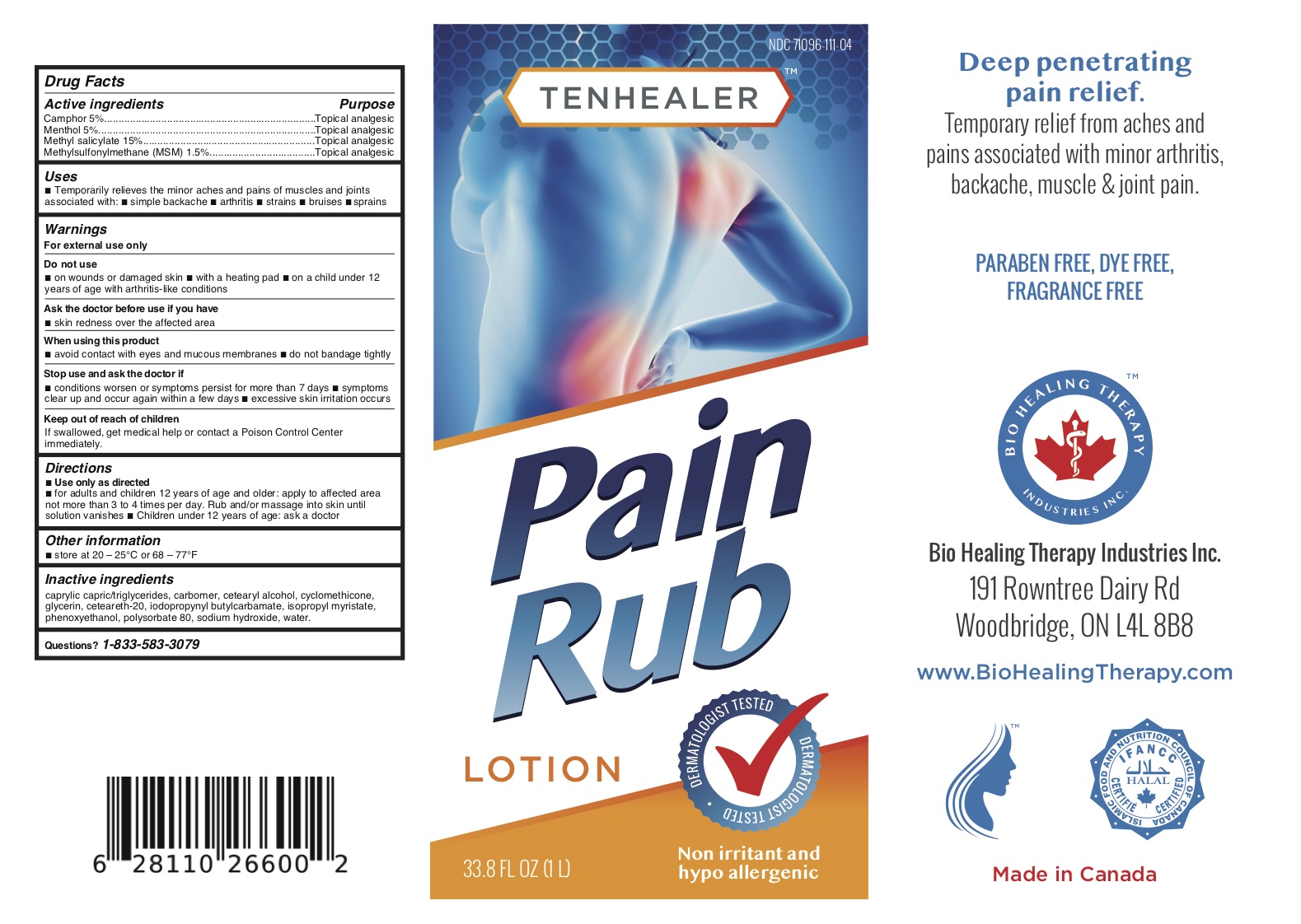
TENHEALER® Pain Rub
Active ingredients...................................Purpose
Camphor 5% .........................................Topical analgesic
Menthol 5% ...........................................Topical analgesic
Methyl salicylate 15% ...........................Topical analgesic
Methylsulfonylmethane (MSM) 1.5% ....Topical analgesic
Uses
temporarily relieves the minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only.
Do not use
- on wounds or damaged skin
- with a heating pad
- on a child under 12 years of age with arthritis-like conditions
Directions
- use only as directed
- adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor
Inactive ingredients
caprylic capric/triglycerides, carbomer, cetearyl alcohol, cyclomethicone, glycerin, ceteareth-20, iodopropynyl butylcarbamate, isopropyl myristate, phenoxyethanol, polysorbate 80, sodium hydroxide, water
Bio Healing Therapy Industries Inc.
191 Rowntree Dairy Rd Woodbridge, ON L4L 8B8
www.BioHealingTherapy.com
Purpose
Active ingredients...................................Purpose
Camphor 5% .........................................Topical analgesic
Menthol 5% ...........................................Topical analgesic
Methyl salicylate 15% ...........................Topical analgesic
Methylsulfonylmethane (MSM) 1.5% ....Topical analgesic
| TENHEALER PAIN RUB
camphor (synthetic), menthol, methyl salicylate lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BIO HEALING THERAPY INDUSTRIES INC (203468038) |
| Registrant - ATS HEALTH AND BEAUTY CARE CORPORATION (241590277) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ATS HEALTH AND BEAUTY CARE CORPORATION | 241590277 | manufacture(71096-111) | |