CHEST CONGESTION RELIEF- guaifenesin tablet, film coated
Cardinal Health 110, LLC. DBA Leader
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Leader 44-532 Delisted
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- cough accompanied by too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- take with a full glass of water
- adults and children 12 years and over: 1 tablet every 4 hours. Do not take more than 6 tablets in 24 hours.
- children under 12 years: do not use
Other information
-
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
FD&C blue #1 aluminum lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, silicon dioxide, sodium starch glycolate, stearic acid
Principal display panel
LEADER™
NDC 70000-0452-2
Chest Congestion Relief
Guaifenesin, 400 mg | Expectorant
Relieves Chest Congestion
Thins and Loosens Mucus
24 IMMEDIATE-RELEASE TABLETS
Actual Size
100% Money Back Guarantee
50844 ORG011853208
© 2019 Cardinal Health. All Rights Reserved. CARDINAL HEALTH,
the Cardinal Health LOGO, LEADER, and the Leader LOGO are
trademarks or registered trademarks of Cardinal Health.
CardinalHealth™
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com 1-800-200-6313
Essential to Care™ since 1979
All LEADER™ Brand Products Have A
100% Money Back Guarantee
Return to place of purchase if not satisfied.
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING
Leader 44-532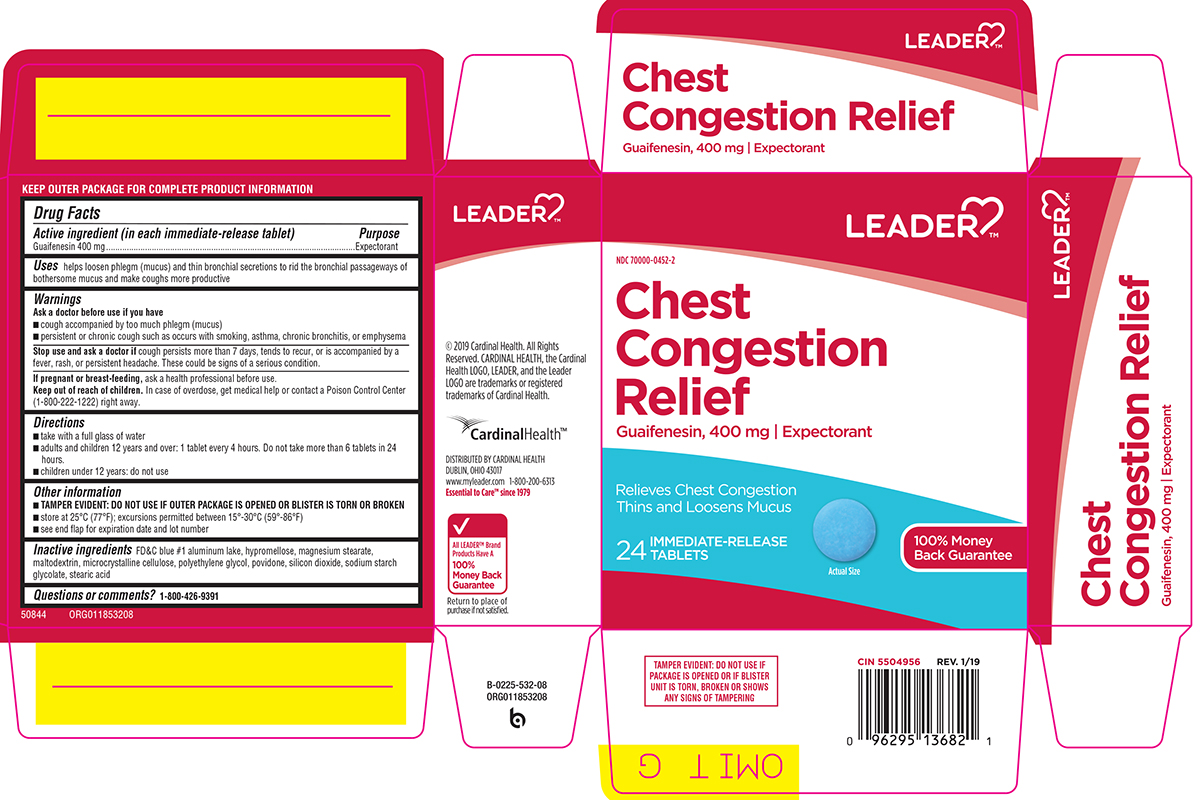
| CHEST CONGESTION RELIEF
guaifenesin tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Cardinal Health 110, LLC. DBA Leader (063997360) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(70000-0452) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(70000-0452) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(70000-0452) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(70000-0452) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(70000-0452) | |