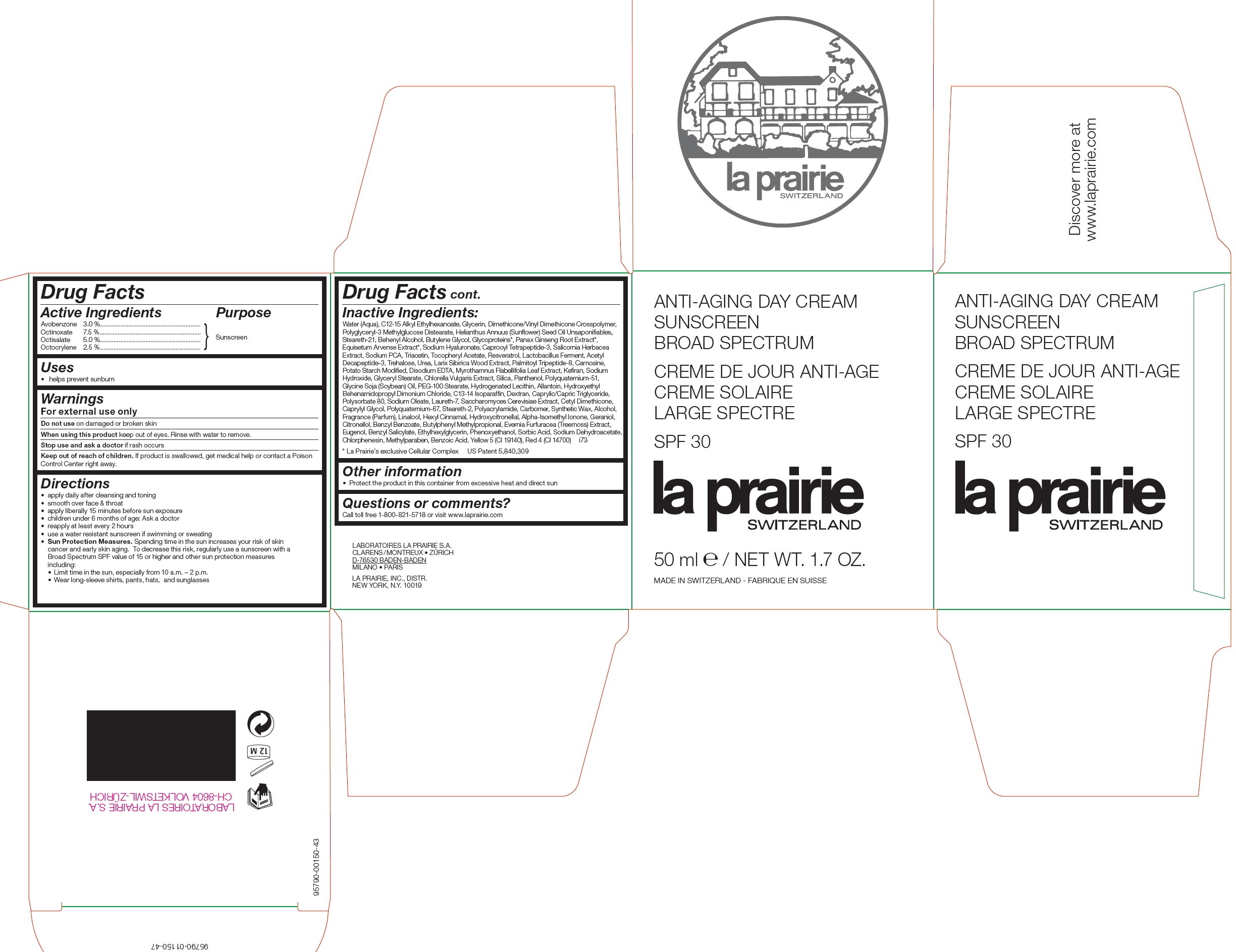
ANTI-AGING DAY SUNSCREEN BROAD SPECTRUM SPF 30- avobenzone, octinoxate, octisalate, octocrylene cream
La Prairie, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ANTI-AGING DAY CREAM SUNSCREEN BROAD SPECTRUM SPF 30
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions:
• apply daily after cleansing and toning • smooth over face and throat • apply liberally 15 minutes before sun exposure • children under 6 months of age: Ask a doctor • reapply at least every 2 hours • use a water resistant sunscreen if swimming or sweating • Sun Protection Measures. Spending time in the sun increase your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • Limit time in the sun, especially from 10 a.m. - 2 p.m. • Wear long-sleeve shirts, pants, hats and sunglasses
Inactive Ingredients:
Water (Aqua), C12-15 Alkyl Ethylhexanoate, Glycerin, Dimethicone/Vinyl Dimethicone Crosspolymer, Polyglyceryl-3 Methylglucose Distearate, Helianthus Annuus (Sunflower) Seed Oil Unsaponifiables, Steareth-21, Behenyl Alcohol, Butylene Glycol, Glycoproteins*, Panax Ginseng Root Extract*, Equisetum Arvense Extract*, Sodium Hyaluronate, Caprooyl Tetrapeptide-3, Salicornia Herbacea Extract, Sodium PCA, Triacetin, Tocopheryl Acetate, Resveratrol, Lactobacillus Ferment, Acetyl Decapeptide-3, Trehalose, Urea, Larix Sibirica Wood Extract, Palmitoyl Tripeptide-8, Carnosine, Potato Starch Modified, Disodium EDTA , Myrothamnus Flabellifolia Leaf Extract, Kefiran, Sodium Hydroxide, Glyceryl Stearate, Chlorella Vulgaris Extract, Silica, Panthenol, Polyquaternium-51, Glycine Soja (Soybean) Oil, PEG-100 Stearate, Hydrogenated Lecithin, Allantoin, Hydroxyethyl Behenamidopropyl Dimonium Chloride, C13-14 Isoparaffin, Dextran, Caprylic/Capric Triglyceride, Polysorbate 80, Sodium Oleate, Laureth-7, Saccharomyces Cerevisiae Extract, Cetyl Dimethicone, Caprylyl Glycol, Polyquaternium-67, Steareth-2, Polyacrylamide, Carbomer, Synthetic Wax, Alcohol, Fragrance (Parfum), Linalool, Hexyl Cinnamal, Hydroxycitronellal, Alpha-Isomethyl Ionone, Geraniol, Citronellol, Benzyl Benzoate, Butylphenyl Methylpropional, Evernia Furfuracea (Treemoss) Extract, Eugenol, Benzyl Salicylate, Ethylhexylglycerin, Phenoxyethanol, Sorbic Acid, Sodium Dehydroacetate, Chlorphenesin, Methylparaben, Benzoic Acid, Yellow 5 (CI 19140), Red 4 (CI 14700) i73 * La Prairie’s exclusive Cellular Complex US Patent 5,840,309
LABORATOIRES LA PRAIRIE S.A.
CLARENS/MONTREUX • ZURICH D-76530 BADEN-BADEN MILANO • PARIS LA PRAIRIE, INC., DISTR. NEW YORK, N.Y. 10019
| ANTI-AGING DAY SUNSCREEN BROAD SPECTRUM SPF 30
avobenzone, octinoxate, octisalate, octocrylene cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - La Prairie, Inc. (606554996) |