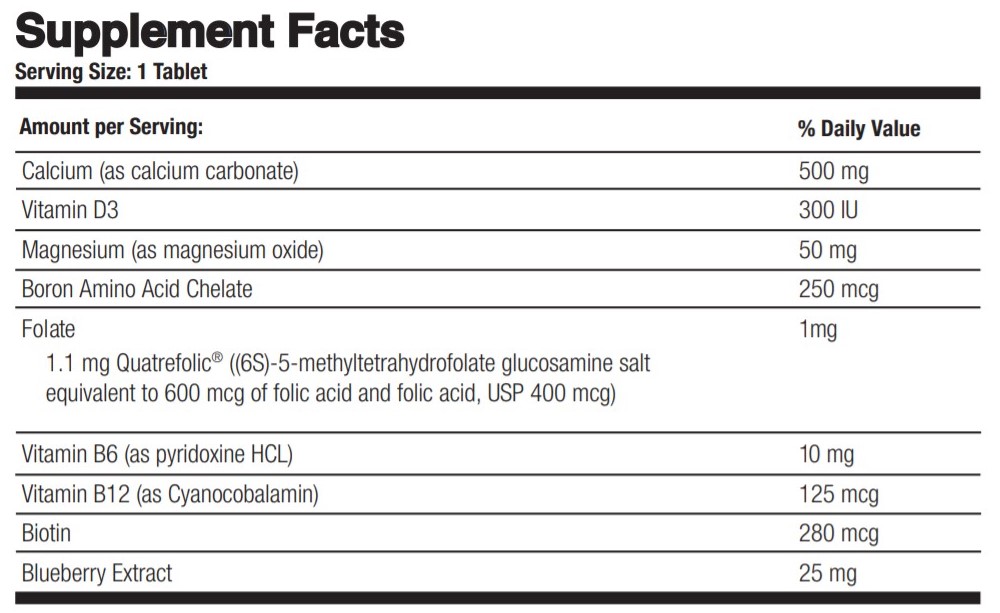
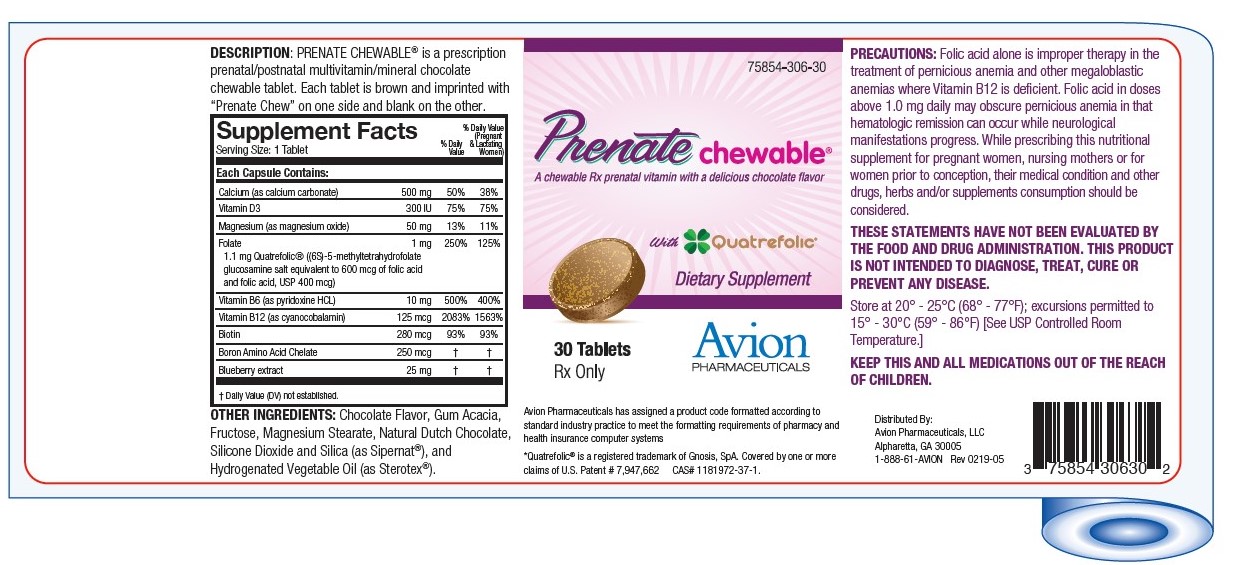
Label: PRENATE CHEWABLE- calcium carbonate, cholecalciferol, magnesium oxide, boron, folic acid, pyridoxine hydrochloride, cyanocobalamin, biotin and bilberry tablet, chewable
- NDC Code(s): 75854-306-30
- Packager: Avion Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 19, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- INACTIVE INGREDIENT
-
INDICATIONS & USAGE
INDICATIONS: PRENATE CHEWABLE ® is a multivitamin/multimineral dietary supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers. PRENATE CHEWABLE ™ can also be beneficial in improving the nutritional status of women prior to conception.
- CONTRAINDICATIONS
-
PRECAUTIONS
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
-
SPL UNCLASSIFIED SECTION
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE.
MANUFACTURED FOR: Avion Pharmaceuticals, LLC
Atlanta, GA 30350
1-888-61-AVION*Quatrefolic ® is a registered trademark of Gnosis, SpA.Covered by one or more claims of U.S. Patent # 7,947,662 CAS# 1181972-37-1
Rev. 0619-05
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRENATE CHEWABLE
calcium carbonate, cholecalciferol, magnesium oxide, boron, folic acid, pyridoxine hydrochloride, cyanocobalamin, biotin and bilberry tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75854-306 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 500 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 300 [iU] MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 50 mg BORON (UNII: N9E3X5056Q) (BORON - UNII:N9E3X5056Q) BORON 250 ug FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 10 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 125 mg BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 280 ug BILBERRY (UNII: 9P2U39H18W) (BILBERRY - UNII:9P2U39H18W) BILBERRY 25 mg Inactive Ingredients Ingredient Name Strength FRUCTOSE (UNII: 6YSS42VSEV) ACACIA (UNII: 5C5403N26O) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color brown Score 2 pieces Shape ROUND Size 22mm Flavor CHOCOLATE Imprint Code PrenateChew Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75854-306-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/16/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/16/2013 Labeler - Avion Pharmaceuticals, LLC (040348516) Establishment Name Address ID/FEI Business Operations Avion Pharmaceuticals, LLC 040348516 manufacture(75854-306)