DEXTROMETHORPHAN HYDROBROMIDE, GUAIFENESIN, AND PHENYLEPHRINE HYDROCHLORIDE- dextromethorphan hydrobromide, guaifenesin, and phenylephrine hydrochloride liquid
Monarch PCM, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
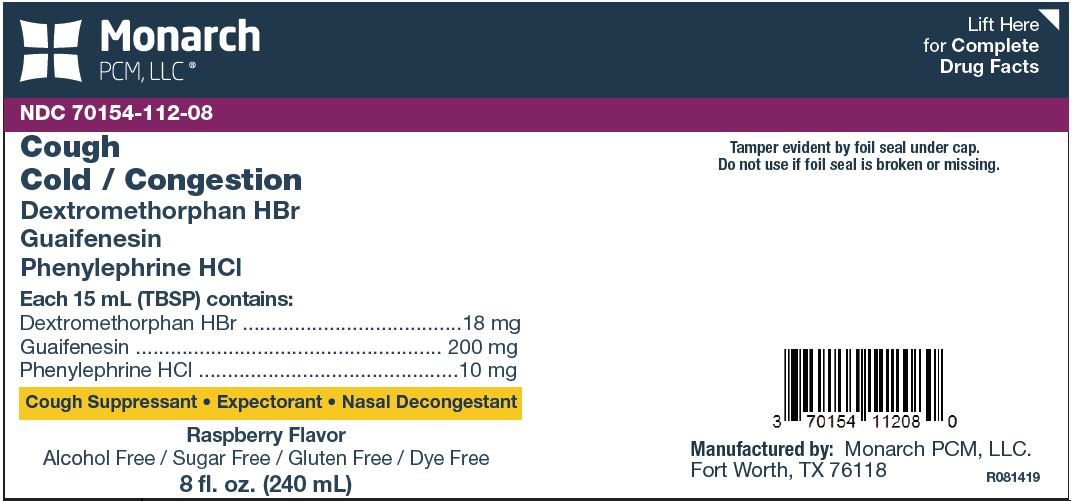
Dextromethorphan HBr, Guaifenesin,and Phenylephrine HCl
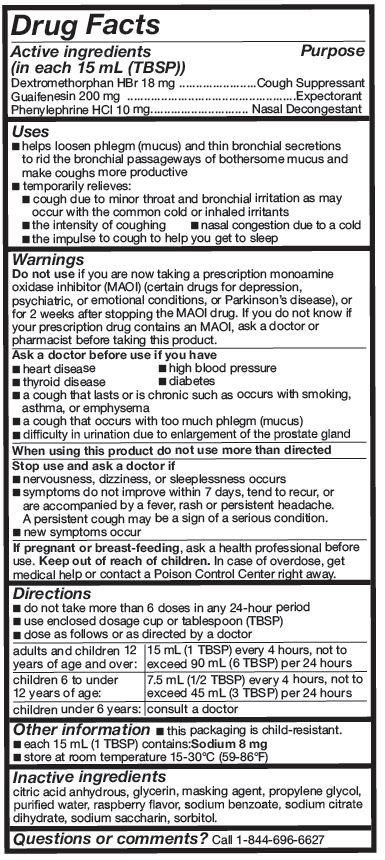
Active ingredients (in each 15 mL (TBSP))
Dextromethorphan HBr 18 mg
Guaifenesin 200 mg
Phenylephrine HCl 10 mg
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
○ cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
○ the intensity of coughing
○ the impulse to cough to help you get to sleep
○ nasal congestion due to a cold
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- a cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
- a cough that occurs with too much phlegm (mucus)
- difficulty in urination due to enlargement of the prostate gland
Directions
- do not take more than 6 doses in any 24-hour period
- use enclosed dosage cup or tablespoon (TBSP)
- dose as follows or as directed by a doctor
| Adults and children 12 years of age and over: | 15 mL (1 TBSP) every 4 hours, not to exceed 90 mL (6 TBSP) iper 24 hours |
| Children 6 to under 12 years of age: | 7.5 mL (1/2 TBSP) every 4 hours, not to exceed 45 mL (3 TBSP) per 24 hours |
| Children under 6 years of age: | Consult a doctor. |
| DEXTROMETHORPHAN HYDROBROMIDE, GUAIFENESIN, AND PHENYLEPHRINE HYDROCHLORIDE
dextromethorphan hydrobromide, guaifenesin, and phenylephrine hydrochloride liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Monarch PCM, LLC. (080000294) |
Revised: 4/2020
Document Id: a473b626-a3e5-7b7c-e053-2995a90ac574
Set id: 90960006-4946-0e18-e053-2a95a90a42ac
Version: 4
Effective Time: 20200429
Monarch PCM, LLC.