M-CLEAR WC- codeine phosphate, guaifenesin liquid
R.A. McNeil Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
M-CLEAR WC
Drug Facts
Active ingredients
(in each 5 mL teaspoonful)
Codeine Phosphate* 6.33 mg
*WARNING: May be habit-forming
Guaifenesin 100 mg
Uses
temporarily relieves these symptoms due to the common cold:
- cough due to minor throat and bronchial irritation
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
Warnings
Do not exceed recommended dosage.
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm (mucus)
- chronic pulmonary disease or shortness of breath, or children who are taking other drugs
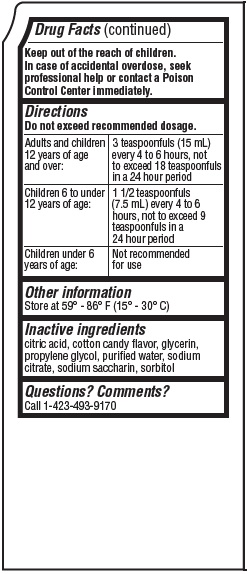
Directions
Do not exceed recommended dosage.
|
Adults and children
|
3 teaspoonfuls (15 mL)
not to exceed 18 teaspoonfuls in a 24 hour period |
| Children 6 to under
12 years of age: |
1 1/2 teaspoonfuls
hours not to exceed 9 teaspoonfuls in a 24 hour period |
|
Children under 6 years of age: |
Not recommended for use |
| M-CLEAR
WC
codeine phosphate, guaifenesin liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - R.A. McNeil Company (008305220) |