FUNGI NAIL TOE AND FOOT- tolnaftate ointment
Denison Pharmaceurticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Fungi Nail Toe and Foot Ointment
Uses
■ Proven effective in the treatment of most athlete’s foot (tinea pedis) and ringworm (tinea corporis). ■ Helps prevent most athlete's foot with daily use. ■ For effective relief of itching, burning and cracking.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
In case of accidental ingestion, contact a physician, emergency medical care facility or Poison Control Center immediately for advice.
Directions
■ Clean affected areas with soap and warm water and dry thoroughly. ■ Apply a thin layer of Fungi-Nail
® Anti-Fungal Ointment over affected area twice daily (morning and night) or as directed by a doctor. ■ Wear well-fitting, ventilated shoes, and change shoes and socks at least once daily. ■ For athlete’s foot pay special attention to spaces between the toes. ■ For athlete’s foot and ringworm, use daily for 4 weeks. ■ To prevent athlete's foot, apply once or twice daily (morning and/or night). ■ For toe fungus, apply under nail and around cuticle area. If condition persists longer, consult a doctor. ■ This product is not effective on the scalp or nails. ■ Supervise children in the use of this product.
Other information
Store at controlled room temperature 15°-30° C (59°-86° F) Protect from freezing. If freezing occurs warm to room temperature.
Inactive ingredients
Aloe Vera Leaf, Carbomer Homopolymer Type A (Allyl Pentaerythritol Crosslinked), Dimethicone 350, Eucalyptol, Lavender Oil, Glyceryl Monostearate, Olive Oil, Phenoxyethanol, Poloxamer 188, Purified Water USP, Sodium Hydroxide, Tea Tree Oil
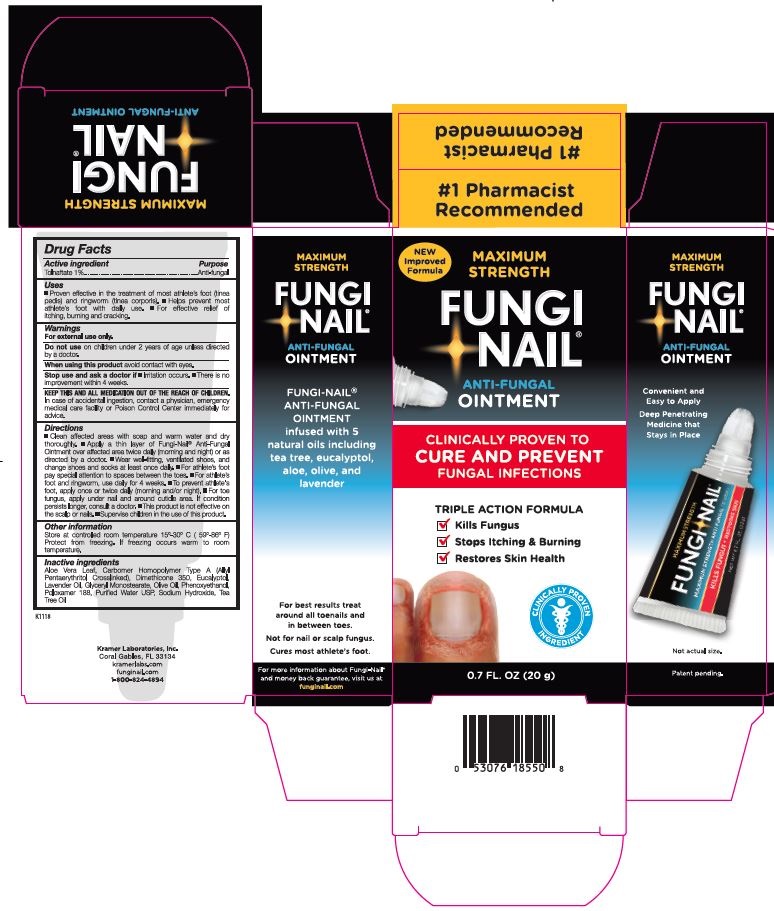
PRINCIPAL DISPLAY PANEL
NEW Improved Formula
MAXIMUM STRENGTH
FUNGI-NAIL
®
ANTI-FUNGAL
OINTMENT
CLINICALLY PROVEN TO
CURE AND PREVENT
FUNGAL INFECTIONS
TRIPLE ACTION FORMULA
✓ Kills Fungus
✓ Stops Itching & Burning
✓ Restores Skin Health
CLINICALLY PROVEN INGREDIENT
0.7 FL. OZ. (20 g)
Convenient and Easy to Apply
Deep Penetrating Medicine that Stays in Place
Not actual size.
Patent pending.
#1 Pharmacist Recommended
FUNGI-NAIL
® ANTI-FUNGAL OINTMENT infused with 5 natural oils including tea tree, eucalyptol, aloe, olive and lavender
For best results treat around all toenails and in between toes.
Not for Nail or scalp fungus.
Cures most athlete's foot.
For more information about Fungi-Nail
® and money back guarantee, visit us at funginail.com
Kramer Laboratories, Inc.
Coral Gables, FL 33134
kramerlabs.com
funginail.com
1-800-824-4894
K1118
MAXIMUM STRENGTH
FUNGI-NAIL
®
ANTI-FUNGAL
OINTMENT
KILLS FUNGUS • RESTORES SKIN
NET WT 0.7 FL. OZ. (20 G)
Distributed By:
KRAMER LABORATORIES
Kramer Laboratories, Coral Gables, FL 33134 U.S.A.
kramerlabs.com
fungi-nail.com
1-800-824-4894
K0918

| FUNGI NAIL TOE AND FOOT
tolnaftate ointment |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Denison Pharmaceurticals, LLC (001207208) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Denison Pharmecuticals | 001207208 | manufacture(0295-8604) | |