GLOPERBA- colchicine solution
Avion Pharmaceuticals, LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GLOPERBA® safely and effectively. See full prescribing information for GLOPERBA®.
GLOPERBA® (colchicine) Oral Solution Initial U.S. Approval: 1961 INDICATIONS AND USAGE
Limitations of use:The safety and effectiveness of GLOPERBA for acute treatment of gout flares during prophylaxis has not been studied. (1) (1) GLOPERBA is not an analgesic medication and should not be used to treat pain from other causes. (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHS0.6 mg/5 mL oral solution ( 3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 7/2019 See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 9/2023 |
FULL PRESCRIBING INFORMATION
1INDICATIONS AND USAGE
GLOPERBA ® (colchicine) Oral Solution is indicated for prophylaxis of gout flares in adults.
Limitations of use: The safety and effectiveness of GLOPERBA for acute treatment of gout flares during prophylaxis has not been studied. GLOPERBA is not an analgesic medication and should not be used to treat pain from other causes.
3DOSAGE FORMS AND STRENGTHS
Ready-to-use solution for oral administration containing 0.6 mg/5 mL of colchicine. The oral solution is a slightly hazy, red liquid with a cherry odor.
4CONTRAINDICATIONS
Patients with renal or hepatic impairment should not be given GLOPERBA in conjunction with drugs that inhibit both CYP3A4 and P-gp [ see Drug Interactions (7)]. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity.
Patients with both renal and hepatic impairment should not be given GLOPERBA.
5WARNINGS AND PRECAUTIONS
5.1Fatal Overdose
Fatal overdoses, both accidental and intentional, have been reported in adults and children who have ingested colchicine [ see Overdosage (10)]. GLOPERBA should be kept out of the reach of children.
5.2Blood Dyscrasias
Myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, pancytopenia and aplastic anemia have been reported with colchicine used in therapeutic doses.
5.3Drug Interactions
Because colchicine is a substrate for both the CYP3A4 metabolizing enzyme and the P-gp efflux transporter, inhibition of either of these pathways may lead to colchicine-related toxicity. Inhibition of both CYP3A4 and P-gp by dual inhibitors (i.e., clarithromycin) has been reported to produce life-threatening or fatal colchicine toxicity due to significant increases in systemic colchicine levels. Therefore, concomitant use of GLOPERBA with inhibitors of both CYP3A4 and P-gp should be avoided. If treatment with colchicine is necessary, a reduced daily dose should be considered and the patient should be closely monitored for colchicine toxicity [ see Drug Interactions (7)].
Use of GLOPERBA in conjunction with drugs that inhibit both CYP3A4 and P-gp is contraindicated in patients with renal or hepatic impairment [ see Contraindications (4)].
5.4Neuromuscular Toxicity
Colchicine-induced neuromuscular toxicity and rhabdomyolysis have been reported with chronic treatment in therapeutic doses, especially in combination with other drugs known to cause this effect. Patients with impaired renal function and elderly patients, even those with normal renal and hepatic function, are at increased risk. Once colchicine treatment is stopped, the symptoms generally resolve within one week to several months.
6ADVERSE REACTIONS
Gastrointestinal disorders are the most common adverse reactions with colchicine. These disorders are often the first signs of toxicity and may indicate that the colchicine dose needs to be reduced or therapy stopped. These disorders include diarrhea, nausea, vomiting, and abdominal pain.
Colchicine has been reported to cause neuromuscular toxicity, which may present as muscle pain or weakness [ see Warnings and Precautions (5.4)].
Toxic manifestations associated with colchicine include myelosuppression, disseminated intravascular coagulation and injury to cells in the renal, hepatic, circulatory and central nervous systems. These toxicities most often occur with excessive accumulation or overdosage [ see Overdosage (10)].
The following adverse reactions have been reported with colchicine. These adverse reactions have been generally reversible upon interrupting treatment or lowering the dose of colchicine.
Neurological: sensory motor neuropathy
Dermatological: alopecia, maculopapular rash, purpura, rash
Digestive: abdominal cramping, abdominal pain, diarrhea, lactose intolerance, nausea, vomiting
Hematological: leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, aplastic anemia
Hepatobiliary: elevated AST, elevated ALT
Musculoskeletal: myopathy, elevated CPK, myotonia, muscle weakness, muscle pain, rhabdomyolysis
Reproductive: azoospermia, oligospermia
7DRUG INTERACTIONS
Colchicine is a substrate of the CYP3A4 metabolizing enzyme and the P-glycoprotein (P-gp) efflux transporter. Fatal drug interactions have been reported when colchicine is administered with clarithromycin, a dual inhibitor of CYP3A4 and P- glycoprotein. Toxicities have also been reported when colchicine is administered with inhibitors of CYP3A4 that may not be potent inhibitors of P-gp (e.g., grapefruit juice, erythromycin, verapamil), or inhibitors of P-gp that may not be potent inhibitors of CYP3A4 (e.g., cyclosporine).
Patients with renal or hepatic impairment should not be given GLOPERBA with drugs that inhibit both CYP3A4 and P-glycoprotein [ seeContraindications(4)]. Combining these dual inhibitors with GLOPERBA in patients with renal and hepatic impairment has resulted in life-threatening or fatal colchicine toxicity.
Physicians should ensure that patients are suitable candidates for treatment with GLOPERBA and remain alert for signs and symptoms of toxic reactions associated with increased colchicine exposure due to drug interactions. Signs and symptoms of colchicine toxicity should be evaluated promptly and, if toxicity is suspected, consider lowering the dose, interruption or discontinuation of GLOPERBA.
7.1CYP3A4
The concomitant use of GLOPERBA and CYP3A4 inhibitors (e.g., clarithromycin, ketoconazole, grapefruit juice, erythromycin, verapamil, etc.) should be avoided due to the potential for serious and life-threatening toxicity [ seeWarnings andPrecautions(5.3) and ClinicalPharmacology(12.3)].
If co-administration of GLOPERBA and a CYP3A4 inhibitor is necessary, the dose of GLOPERBA should be adjusted by either reducing the daily dose or reducing the dose frequency, and the patient should be monitored carefully for colchicine toxicity [ seeClinicalPharmacology(12.3)].
7.2P- Glycoprotein
The concomitant use of GLOPERBA and inhibitors of P-glycoprotein (e.g. clarithromycin, ketoconazole, cyclosporine, etc.) should be avoided due to the potential for serious and life-threatening toxicity [ seeWarnings andPrecautions(5.3) and ClinicalPharmacology(12.3)].
If co-administration of GLOPERBA and a P-gp inhibitor is necessary, the dose of GLOPERBA should be adjusted by either reducing the daily dose or reducing the dose frequency, and the patient should be monitored carefully for colchicine toxicity [ seeClinicalPharmacology(12.3)].
7.3HMG- CoA Reductase Inhibitors and Fibrates
Some drugs such as HMG-CoA reductase inhibitors and fibrates may increase the risk of myopathy when combined with GLOPERBA. Complaints of muscle pain or weakness could be an indication to check serum creatinine kinase levels for signs of myopathy.
7.4Drug Interaction Studies
Two pharmacokinetic studies evaluated the effects of co-administration of posaconazole (300 mg QD), ciprofloxacin (500 mg BID), amlodipine (5 to 10 mg QD), and carvedilol (20 to 40 mg QD) on the systemic levels of colchicine.
GLOPERBA can be administered with amlodipine, carvedilol, and ciprofloxacin at the tested doses without a need for dose adjustment. However, the results should not be extrapolated to other co-administered drugs. Colchicine plasma levels were markedly elevated when GLOPERBA was co-administered with posaconazole. The recommended dose of GLOPERBA when co-administered with posaconazole is 0.24 mg (2 mL).
8USE IN SPECIFIC POPULATIONS
8.1Pregnancy
Risk Summary
Available human data from published literature on colchicine use in pregnancy over several decades have not identified any drug associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). Although animal reproduction and development studies were not conducted with GLOPERBA, published animal reproduction and development studies indicate that colchicine causes embryofetal toxicity and altered postnatal development at exposures within or above the clinical therapeutic range.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Available data from published observational studies, case series, and case reports over several decades do not suggest an increased risk for major birth defects or miscarriage in pregnant women with rheumatic diseases (such as rheumatoid arthritis, Behçet’s disease, or familial Mediterranean fever (FMF)) treated with colchicine at therapeutic doses during pregnancy. Limitations of these data include the lack of randomization and inability to control for confounders such as underlying maternal disease and maternal use of concomitant medications.
8.2Lactation
Risk Summary
Colchicine is present in human milk (see Data). Adverse events in breastfed infants have not been reported in the published literature after administration of colchicine to lactating women. There are no data on the effects of colchicine on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for GLOPERBA and any potential adverse effects on the breastfed infant from GLOPERBA or from the underlying maternal condition .
Data
Limited published data from case reports and a small lactation study demonstrate that colchicine is present in breastmilk. A systematic review of literature reported no adverse effects in 149 breastfed children and advised to reconsider breastfeeding if the infant has diarrhea. In a prospective observational cohort study, no gastrointestinal or other symptoms were reported in 38 colchicine-exposed breastfed infants.
8.3Females and Males of Reproductive Potential
Infertility
Case reports and epidemiology studies in human male subjects on colchicine therapy indicate that infertility from colchicine is rare and may be reversible.
8.4Pediatric Use
Gout is rare in pediatric patients; safety and effectiveness of GLOPERBA in pediatric patients has not been established.
8.5Geriatric Use
Because of the increased incidence of decreased renal function in the elderly population, and the higher incidence of other co-morbid conditions in the elderly population requiring the use of other medications, reducing the dosage of colchicine when elderly patients are treated with colchicine should be carefully considered [ see Clinical Pharmacology (12.3)].
8.6Renal Impairment
No dedicated pharmacokinetic study has been conducted using GLOPERBA in patients with varying degrees of renal impairment. Colchicine is known to be excreted in urine in humans and the presence of severe renal impairment has been associated with colchicine toxicity. Urinary clearance of colchicine and its metabolites may be decreased in patients with impaired renal function. Dose reduction or alternatives should be considered for the prophylaxis of gout flares in patients with severe renal impairment. Colchicine is not effectively removed by hemodialysis. Patients who are undergoing hemodialysis should be monitored carefully for colchicine toxicity.
8.7Hepatic Impairment
No dedicated pharmacokinetic study using GLOPERBA has been conducted in patients with varying degrees of hepatic impairment. Colchicine is known to be metabolized in humans and the presence of severe hepatic impairment has been associated with colchicine toxicity. Hepatic clearance of colchicine may be significantly reduced and plasma half-life prolonged in patients with chronic hepatic impairment.
Dose reduction or alternatives should be considered for the prophylaxis of gout flares in patients with severe hepatic impairment.
10OVERDOSAGE
The dose of colchicine that would induce significant toxicity for an individual is unknown. Fatalities have occurred after ingestion of a dose as low as 7 mg over a four-day period, while other patients have survived after ingesting more than 60 mg. A review of 150 patients who overdosed on colchicine found that those who ingested less than 0.5 mg/kg survived and tended to have milder adverse reactions such as gastrointestinal symptoms, whereas those who took 0.5 to 0.8 mg/kg had more severe adverse reactions, including myelosuppression. There was 100% mortality in those who ingested more than 0.8 mg/kg.
- The first stage of acute colchicine toxicity typically begins within 24 hours of ingestion and includes gastrointestinal symptoms such as abdominal pain, nausea, vomiting, diarrhea and significant fluid loss, leading to volume depletion. Peripheral leukocytosis may also be seen.
- Life-threatening complications occur during the second stage, which occurs 24 to 72 hours after drug administration, attributed to multiorgan failure and its consequences. Death is usually a result of respiratory depression and cardiovascular collapse. If the patient survives, recovery of multiorgan injury may be accompanied by rebound leukocytosis and alopecia starting about one week after the initial ingestion.
- Treatment of colchicine poisoning should begin with gastric lavage and measures to prevent shock. Otherwise, treatment is symptomatic and supportive. No specific antidote is known. Colchicine is not effectively removed by hemodialysis [see Clinical Pharmacology (12.3)].
11DESCRIPTION
Colchicine is an alkaloid obtained from various species of Colchicum. The chemical name for colchicine is ( S)- N-(5,6,7,9- tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[ a]heptalen-7-yl) acetamide with a molecular formula of C 22H 25NO 6 and a molecular weight of 399.4. The structural formula of colchicine is provided in Figure 1.
Figure 1: Colchicine Structural Formula
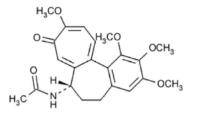
Colchicine consists of pale yellow scales or powder; it darkens on exposure to light. Colchicine is soluble in water, freely soluble in alcohol, and slightly soluble in ether.
GLOPERBA is supplied for oral administration as a slightly hazy, red liquid with a cherry odor, containing 0.6 mg/5 mL of the active ingredient colchicine USP. Inactive ingredients: benzyl alcohol, FD&C Red No. 40, artificial cherry flavor, anhydrous citric acid, dibasic sodium phosphate, glycerin, propylene glycol, sucralose, xanthan gum and purified water.
12CLINICAL PHARMACOLOGY
12.1Mechanism of Action
Colchicine’s effectiveness as a prophylactic treatment for gout has been postulated to be due to its ability to block neutrophil-mediated inflammatory responses induced by monosodium urate crystals in synovial fluid. Colchicine disrupts the polymerization of β-tubulin into microtubules, thereby preventing the activation, degranulation and migration of neutrophils to sites of inflammation. Colchicine also interferes with the inflammasome complex found in neutrophils and monocytes that mediates interleukin-1β (IL-1β) activation.
12.3Pharmacokinetics
Absorption
In healthy adults, GLOPERBA reached a mean C max of 2.16 ± 0.87 ng/mL in 1 hour (range 0.5 to 2 hours) after a single dose administered under fasting conditions. A minimal food effect was observed when GLOPERBA was administered following a high fat, high calorie meal. A slight decrease in C max was observed; however, the overall extent of absorption based on AUC 0- t and AUC 0- inf, was similar in the fed and fasted states. The absolute bioavailability of colchicine is reported to be approximately 45%. Mean pharmacokinetic parameter values for GLOPERBA in healthy adults are shown in Table1.
| Table 1: Mean Pharmacokinetic Parameter Estimates for GLOPERBA in Healthy Adults | ||
| Parameter | GLOPERBA, 0.6 mg
(0.12 mg/mL, 5 mL) Fasted (N=34) | GLOPERBA, 0.6 mg
(0.12 mg/mL, 5 mL) Fed (N=34) |
| C max (ng/mL) | 2.16 (0.87) | 1.68 (0.39) |
| AUC 0- t (h∙ng/mL) | 18.59 (4.64) | 17.20 (4.23) |
| AUC 0- inf (h∙ng/mL) | 19.90 (4.74) | 18.47 (4.29) |
| T max (h) (Min-Max) | 1.00 (0.50: 2.00) | 2.00 (1.00: 4.00) |
| t 1/2 (h) | 31.04 (5.99) | 30.54 (5.22) |
Distribution
The mean apparent volume of distribution(V z/F) of GLOPERBA in healthy adults was approximately 1420 L. Colchicine binding to serum protein is reported to be low (39%) primarily due to albumin regardless of concentration.
Colchicine crosses the placenta (plasma levels in the fetus are reported to be approximately 15% of the maternal concentration) [s ee Use in Specific Populations (8.1)]. Colchicine also distributes into breast milk at concentrations similar to those found in the maternal serum [s ee Use in Specific Populations (8.2)].
Metabolism
In vitro studies using human liver microsomes have shown that CYP3A4 is involved in the metabolism of colchicine to 2- O-demethylcolchicine (2-DMC) and 3- O-demethylcolchicine (3-DMC). Glucuronidation is also believed to be a metabolic pathway for colchicine.
Elimination
The mean elimination half-life of GLOPERBA in healthy adults is 31 hours (± 6 hours). In a published study in healthy adults approximately 40 to 65% of a single 1-mg oral dose of colchicine was reported to be recovered unchanged in urine. Enterohepatic recirculation and biliary excretion are postulated to play a role in colchicine elimination. Colchicine is also a substrate of P-gp, and P-gp efflux is postulated to play an important role in colchicine disposition.
Specific Populations
There is no difference between men and women in the pharmacokinetic disposition of colchicine.
Pediatric Patients: Pharmacokinetics of colchicine were not evaluated in pediatric patients.
Geriatric Patients: Pharmacokinetics of GLOPERBA have not been determined in elderly patients. A published report described the pharmacokinetics of a 1-mg oral colchicine tablet dose in four elderly women compared to six young healthy males. The mean age of the four elderly women was 83 years (range 75 to 93), mean weight was 47 kg (38 to 61 kg) and mean creatinine clearance was 46 mL/minute (range 25 to 75 mL/minute). Mean peak plasma levels and AUC of colchicine were two times higher in elderly subjects compared to young healthy males. It is possible that the higher exposure in the elderly subjects was due to decreased renal function.
Patients with Renal Impairment: Pharmacokinetics of colchicine in patients with mild and moderate renal impairment is not known. A published report described the disposition of colchicine (1 mg) in young adult men and women patients who had end-stage renal disease requiring dialysis compared to patients with normal renal function. Patients with end-stage renal disease had 75% lower colchicine clearance (0.17 vs. 0.73 L/hr/kg) and prolonged plasma elimination half-life (18.8 hours vs. 4.4 hours) as compared to subjects with normal renal function [s ee Use in Specific Populations(8.6)].
Patients with Hepatic Impairment: Published reports on the pharmacokinetics of intravenous colchicine in patients with severe chronic liver disease, as well as those with alcoholic or primary biliary cirrhosis, and normal renal function suggest wide inter-patient variability. In some subjects with mild to moderate cirrhosis, the clearance of colchicine is significantly reduced and plasma half-life prolonged compared to healthy subjects. In subjects with primary biliary cirrhosis, no consistent trends were noted [s ee Use in Specific Populations (8.7)]. No pharmacokinetic data are available for patients with severe hepatic impairment (Child-Pugh C).
Drug Interactions
Pharmacokinetic studies evaluating changes in systemic levels of colchicine when co-administered with CYP3A4 and P-gp inhibitors in healthy subjects have been conducted with GOLPERBA. Carvedilol 20 to 40 mg QD (considered a P-gp inhibitor), amlodipine 5 to 10 mg QD (considered a weak inhibitor of CYP3A4) and ciprofloxacin 500 mg BID (considered a moderate CYP3A4 inhibitor) did not cause any significant changes in colchicine systemic levels. Co-administration with posaconazole 300 mg QD (considered a strong CYP3A4 inhibitor) increased AUC by approximately 3-fold.
These results should not be extrapolated to other inhibitors as colchicine is known to be a substrate for CYP3A4 and P-gp, and case reports of colchicine toxicity associated with the co-administration of CYP3A4 and P-gp inhibitors (i.e., clarithromycin) have been published.
Fatal drug interactions have been reported when colchicine is administered with clarithromycin, a dual inhibitor of CYP3A4 and P-gp. Toxicities have also been reported when colchicine is administered with inhibitors of CYP3A4 that may not be potent inhibitors of P-gp (e.g., grapefruit juice), or inhibitors of P-gp that may not be potent inhibitors of CYP3A4 (e.g., cyclosporine). If treatment with a P-gp and CYP3A4 inhibitor is required in patients with normal renal and hepatic function, the patients’ dose of colchicine may need to be reduced or interrupted.
13NONCLINICAL TOXICOLOGY
13.1Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies of colchicine have not been conducted. Due to the potential for colchicine to produce aneuploid cells (cells with an unequal number of chromosomes), colchicine presents a theoretical increased risk of malignancy.
Mutagenesis
Colchicine was negative for mutagenicity in the bacterial reverse mutation assay. In a chromosomal aberration assay in cultured human white blood cells, colchicine treatment resulted in the formation of micronuclei. Since published studies demonstrated that colchicine induces aneuploidy from the process of mitotic nondisjunction without structural DNA changes, colchicine is not considered clastogenic, although micronuclei are formed.
Impairment of Fertility
There were no studies conducted of the effects of GLOPERBA on fertility. Published nonclinical studies have demonstrated that colchicine-induced disruption of microtubule formation affects meiosis and mitosis. Published colchicine reproductive studies have reported abnormal sperm morphology and reduced sperm counts in males and interference with sperm penetration, second meiotic division and normal cleavage in females.
14CLINICAL STUDIES
The evidence for the efficacy of colchicine in patients with chronic gout is derived from the published literature. Two randomized clinical trials assessed the efficacy of colchicine 0.6 mg twice a day for the prophylaxis of gout flares in patients with gout initiating treatment with urate-lowering therapy. In both trials, treatment with colchicine decreased the frequency of gout flares.
16HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Medication Guide).
Dosing Instructions
Patients should be advised to take GLOPERBA as prescribed, even if they are feeling better. Patients should not alter the dose or discontinue treatment without consulting with their doctor. If a dose of GLOPERBA is missed, take the dose as soon as possible and then return to the normal dosing schedule. However, if a dose is skipped the patient should not double the next dose.
Advise patients and caregivers to measure GLOPERBA with an accurate milliliter measuring device. A household teaspoon is not an accurate measuring device. Advise patients and caregivers to ask their pharmacist to recommend an appropriate measuring device and for instructions for measuring the correct dose.
Fatal Overdose
Instruct patient that fatal overdoses, both accidental and intentional, have been reported in adults and children who have ingested colchicine. GLOPERBA should be kept out of the reach of children.
Blood Dyscrasias
Patients should be informed that bone marrow depression with agranulocytosis, aplastic anemia and thrombocytopenia may occur with GLOPERBA.
Drug and Food Interactions
Advise patients that many drugs or other substances may interact with GLOPERBA and some interactions could be fatal. Therefore, patients should report to their healthcare provider all of the current medications they are taking and check with their healthcare provider before starting any new medications, including short-term medications such as antibiotics. Patients should also be advised to report the use of nonprescription medication or herbal products. Grapefruit and grapefruit juice may also interact and should not be consumed during GLOPERBA treatment.
Neuromuscular Toxicity
Patients should be informed that muscle pain or weakness, tingling or numbness in fingers or toes may occur with GLOPERBA alone or when it is used with certain other drugs. Patients developing any of these signs or symptoms must discontinue GLOPERBA and seek medical evaluation immediately.
Infertility
Advise males of reproductive potential that GLOPERBA may rarely and transiently impair fertility [see Use in Specific Populations (8.3)].
RxONLY
Manufacture for:
Avion Pharmaceuticals, LLC
Alpharetta, GA 30005
L-0003 Rev 0719-01
| MEDICATION GUIDE
GLOPERBA® (Glow per’ bah) (colchicine) Oral Solution |
|||
| What is the most important information I should know about GLOPERBA?
GLOPERBA can cause serious side effects or death if levels of GLOPERBA are too high in your body.
|
|||
| What is GLOPERBA?
GLOPERBA is a prescription medicine used to prevent gout flares in adults. It is not known if GLOPERBA is safe and effective for the treatment of sudden (acute) gout flares. GLOPERBA is not a pain medicine, and it should not be taken to treat pain related to other medical conditions unless specifically prescribed for those conditions. It is not known if GLOPERBA is safe and effective in children. |
|||
Do not take GLOPERBA if you:
|
|||
Before taking GLOPERBA, tell your healthcare provider about all of your medical conditions, including if you:
|
|||
How should I take GLOPERBA?
|
|||
| What should I avoid while taking GLOPERBA?
Avoid eating grapefruit or drinking grapefruit juice while taking GLOPERBA. It can increase your chances of having serious side effects. |
|||
| What are the possible side effects of GLOPERBA?
GLOPERBA can cause serious side effects, including: See " What is the most important information that I should know about GLOPERBA?"
|
|||
| ○ pale or gray color to your lips, tongue or palms of your hands. | ○ feel weak or tired | ||
| ○ unusual bleeding or bruising | ○ increased infections | ||
|
|||
| ○ muscle weakness or pain | ○numbness or tingling in your fingers or toes | ||
| The most common side effects of GLOPERBA include: | |||
| ○ diarrhea | ○ nausea | ○ vomiting | ○ abdominal (stomach) pain |
| Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of GLOPERBA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
How should I store GLOPERBA?
|
|||
| General information about the safe and effective use of GLOPERBA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use GLOPERBA for a condition for which it was not prescribed. Do not give GLOPERBA to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about GLOPERBA that is written for healthcare professionals. |
|||
| What are the ingredients in GLOPERBA?
Active Ingredient: colchicine Inactive Ingredients: benzyl alcohol, FD&C Red No. 40, artificial cherry flavor, anhydrous citric acid, dibasic sodium phosphate, glycerin, propylene glycol, sucralose, xanthan gum and purified water. |
|||
| Rx ONLY | |||
|
Manufactured for:
Alpharetta, GA 30005 |
|||
| For more information, call 1-888-61-AVION | |||
| This Medication Guide has been approved by the U.S Food and Drug Administration Issued: 7/2019 | |||
| GLOPERBA
colchicine solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
 |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Avion Pharmaceuticals, LLC (040348516) |
| Registrant - Avion Pharmaceuticals, LLC (040348516) |