Label: MEGESTROL ACETATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 53808-0614-1 - Packager: State of Florida DOH Central Pharmacy
- This is a repackaged label.
- Source NDC Code(s): 0054-8604
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated June 7, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
Description
Megestrol acetate, is a synthetic, antineoplastic and progestational drug. Megestrol acetate is a white, crystalline solid chemically designated as pregna-4,6-diene-3,20-dione, 17-(acetyloxy)-6-methyl-. Solubility at 37°C in water is 2 mcg per mL, solubility in plasma is 24 mcg per mL. Its molecular weight is 384.51. The empirical formula is C24 H32O4 and the structural formula is represented as follows:
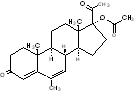
megesterol acetate, USP
Megestrol acetate tablets are supplied for oral administration containing 20 mg or 40 mg megestrol acetate and the following inactive ingredients: acacia, colloidal silicon dioxide, dibasic calcium phosphate, lactose monohydrate, magnesium stearate, and starch (corn).
-
Clinical Pharmacology
While the precise mechanism by which megestrol produces its antineoplastic effects against endometrial carcinoma is unknown at the present time, inhibition of pituitary gonadotrophin production and resultant decrease in estrogen secretion may be factors. There is evidence to suggest a local effect as a result of the marked changes brought about by the direct instillation of progestational agents into the endometrial cavity. The antineoplastic action of megestrol acetate on carcinoma of the breast is effected by modifying the action of other steroid hormones and by exerting a direct cytotoxic effect on tumor cells. In metastatic cancer, hormone receptors may be present in some tissues but not others. The receptor mechanism is a cyclic process whereby estrogen produced by the ovaries enters the target cell, forms a complex with cytoplasmic receptor and is transported into the cell nucleus. There it induces gene transcription and leads to the alteration of normal cell functions. Pharmacologic doses of megestrol acetate not only decrease the number of hormone-dependent human breast cancer cells but also are capable of modifying and abolishing the stimulatory effects of estrogen on these cells. It has been suggested that progestins may inhibit in one of two ways: by interfering with either the stability, availability, or turnover of the estrogen receptor complex in its interaction with genes or in conjunction with the progestin receptor complex, by interacting directly with the genome to turn off specific estrogen-responsive genes.
There are several analytical methods used to estimate megestrol acetate plasma levels, including mass fragmentography, gas chromatography (GC), high pressure liquid chromatography (HPLC) and radioimmunoassay. The plasma levels by HPLC assay or radioimmunoassay methods are about one-sixth those obtained by the GC method. The plasma levels are dependent not only on the method used, but also on intestinal and hepatic inactivation of the drug, which may be affected by factors such as intestinal tract motility, intestinal bacteria, antibiotics administered, body weight, diet, and liver function.
Metabolites account for only 5% to 8% of the administered dose and are considered negligible. The major route of drug elimination in humans is the urine. When radiolabeled megestrol acetate was administered to humans in doses of 4 to 90 mg, the urinary excretion within 10 days ranged from 56.5% to 78.4% (mean 66.4%) and fecal excretion ranged from 7.7% to 30.3% (mean 19.8%). The total recovered radioactivity varied between 83.1% and 94.7% (mean 86.2%). Respiratory excretion as labeled carbon dioxide and fat storage may have accounted for at least part of the radioactivity not found in the urine and feces.
In normal male volunteers (n=23) who received 160 mg of megestrol acetate given as a 40 mg q.i.d. regimen, the oral absorption of megestrol acetate appeared to be variable. Plasma levels were assayed by a high pressure liquid chromatograpic (HPLC) procedure. Peak drug levels for the first 40 mg dose ranged from 10 to 56 ng/mL (mean 27.6 ng/mL) and the times to peak concentrations ranged from 1.0 to 3.0 hours (mean 2.2 hours). Plasma elimination half-life ranged from 13.0 to 104.9 hours (mean 34.2 hours). The steady state plasma concentrations for a 40 mg q.i.d. regimen have not been established.
- Indications and Usage
- Contraindications
-
Warnings
Megestrol acetate may cause fetal harm when administered to a pregnant woman. Fertility and reproduction studies with high doses of megestrol acetate have shown a reversible feminizing effect on some male rat fetuses. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking (receiving) this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
The use of megestrol in other types of neoplastic disease is not recommended.
(See also Precautions: Carcinogenesis, Mutagenesis, and Impairment of Fertility section.)
The glucocorticoid activity of megestrol acetate tablets has not been fully evaluated. Clinical cases of new onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and overt Cushing’s syndrome have been reported in association with the chronic use of megestrol. In addition, clinical cases of adrenal insufficiency have been observed in patients receiving or being withdrawn from chronic megestrol therapy in the stressed and non-stressed state. Furthermore, adrenocorticotropin (ACTH) stimulation testing has revealed the frequent occurrence of asymptomatic pituitary-adrenal suppression in patients treated with chronic megestrol therapy. Therefore, the possibility of adrenal insufficiency should be considered in any patient receiving or being withdrawn from chronic megestrol therapy who presents with symptoms and/or signs suggestive of hypoadrenalism (e.g., hypotension, nausea, vomiting, dizziness, or weakness) in either the stressed or non-stressed state. Laboratory evaluation for adrenal insufficiency and consideration of replacement or stress doses of a rapidly acting glucocorticoid are strongly recommended in such patients. Failure to recognize inhibition of the hypothalamic-pituitary-adrenal axis may result in death. Finally, in patients who are receiving or being withdrawn from chronic megestrol therapy, consideration should be given to the use of empiric therapy with stress doses of a rapidly acting glucocorticoid in conditions of stress or serious intercurrent illness (eg., surgery, infection).
-
Precautions
General
Close surveillance is indicated for any patient treated for recurrent or metastatic cancer. Use with caution in patients with a history of thromboembolic disease.
Use in Diabetics
Exacerbation of pre-existing diabetes with increased insulin requirements has been reported in association with the use of megestrol acetate.
Information for the Patients
Patients using megestrol acetate should receive the following instructions:
- This medication is to be used as directed by the physician.
- Report any adverse reaction experiences while taking this medication.
Laboratory Tests
Breast malignancies in which estrogen and/or progesterone receptors are positive are more likely to respond to megestrol.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Administration of megestrol acetate to female dogs for up to 7 years is associated with an increased incidence of both benign and malignant tumors of the breast. Comparable studies in rats and studies in monkeys are not associated with an increased incidence of tumors. The relationship of the dog tumors to humans is unknown but should be considered in assessing the benefit-to-risk ratio when prescribing megestrol acetate and in surveillance of patients on therapy (see Warnings section).
Nursing Mothers
Because of the potential for adverse effects on the newborn, nursing should be discontinued if megestrol is required for treatment of cancer.
Geriatric Use
Insufficient data from clinical studies of megesterol acetate tablets are available for patients 65 years of age and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Megestrol acetate is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
Adverse Reactions
Weight Gain
Weight gain is a frequent side effect of megestrol. This gain has been associated with increased appetite and is not necessarily associated with fluid retention.
Thromboembolic Phenomena
Thromboembolic phenomena including thrombophlebitis and pulmonary embolism (in some cases fatal) have been reported.
Other Adverse Reactions
Heart failure, nausea and vomiting, edema, breakthrough menstrual bleeding, dyspnea, tumor flare (with or without hypercalcemia), hyperglycemia, glucose intolerance, alopecia, hypertension, carpal tunnel syndrome, mood changes, hot flashes, malaise, asthenia, lethargy, sweating and rash.
-
Overdosage
No serious unexpected side effects have resulted from studies involving megestrol acetate administered in dosages as high as 1600 mg/day. Oral administration of large, single doses of megestrol acetate (5 g/kg) did not produce toxic effects in mice. Megestrol acetate has not been tested for dialyzability; however, due to its low solubility it is postulated that this would not be an effective means of treating overdose.
- Dosage and Administration
-
How Supplied
Megestrol Acetate Tablets, USP
20 mg white, round, scored tablets
(Identified 54 763)
40 mg white, round, scored tablets
(Identified 54 352)
They are supplied by State of Florida DOH Central Pharmacy as follows:
NDC Strength Quantity/Form Color Source Prod. Code 53808-0614-1 40 mg 30 Tablets in a Blister Pack WHITE 0054-8604 Dispense in a well-closed container
as defined in the USP/NF.
- STORAGE
-
SPECIAL HANDLING
Health Hazard Data
There is no threshold limit value established by OSHA, NIOSH, or ACGIH.
Exposure or “overdose” at levels approaching recommended dosing levels could result in side effects described above (see Warnings and Adverse Reactions sections). Women at risk of pregnancy should avoid such exposure.
© RLI, 2007
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
- Label Image for 40mg
-
INGREDIENTS AND APPEARANCE
MEGESTROL ACETATE
megestrol acetate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53808-0614(NDC:0054-8604) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEGESTROL ACETATE (UNII: TJ2M0FR8ES) (MEGESTROL - UNII:EA6LD1M70M) MEGESTROL ACETATE 40 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE Score 2 pieces Shape ROUND (flat faced, beveled edges) Size 8mm Flavor Imprint Code 54;352 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53808-0614-1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074458 07/01/2009 Labeler - State of Florida DOH Central Pharmacy (829348114) Establishment Name Address ID/FEI Business Operations State of Florida DOH Central Pharmacy 829348114 repack


