Eyewash
Active ingredient
Sterile Water 99%
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Stop use and ask a doctor if
- you experience eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Eyewash
Inactive ingredients
sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic
Eyewash
Questions
1-800-430-5490
Aspirin
Active ingredient (in each tablet)
Aspirin 325 mg (NSAID)* *nonsteroidal anti-inflammatory drug
Aspirin
Purpose
Pain reliever/fever reducer
Aspirin
Uses
temporarily reduces fever and relieves minor aches and pains associated with:
- a cold
- headache
- toothache
- muscular aches
- backache
- minor pain of arthritis
- premenstrual and menstrual periods
Aspirin
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:are:
- age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you are allergic to aspirin or any other pain reliever/fever reducer
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if you are
- taking a prescription drug for diabetes, gout or arthritis
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- ringing in the ears or loss of hearing occurs
- any new symptoms appear
If pregnant or breast-feeding,
If pregnant or breat-feeding, ask a health professional before use. It is especially important not to use aspirin during the last three months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children.
- In case of overdose, get medical help or contact Poison Control Center right away.
Aspirin
Directions
- drink a full glass of water with each dose
- adults and children 12 years of age and older: take 1 or 2 tablets every 4 hours while symptoms last, not more than 12 tablets in 24 hours
- children under 12 years of age: consult a doctor
Aspirin
Other information
- store at room temperature 15° - 30°C (59° - 86°F)
- TAMPER EVIDIENT PACKETS
- DO NOT USE IF OPEN OR TORN
Aspirin
Inactive ingredients
corn starch, croscarmellose sodium*, hypromellose*, microcrystalline cellulose*, mineral oil*, polyethylene glycol*, povidone, propylene glycol, silicon dioxide, stearic acid*, titanium dioxide*
*may contain these ingredients
Aspirin
Questions or Comments
1-800-430-5490
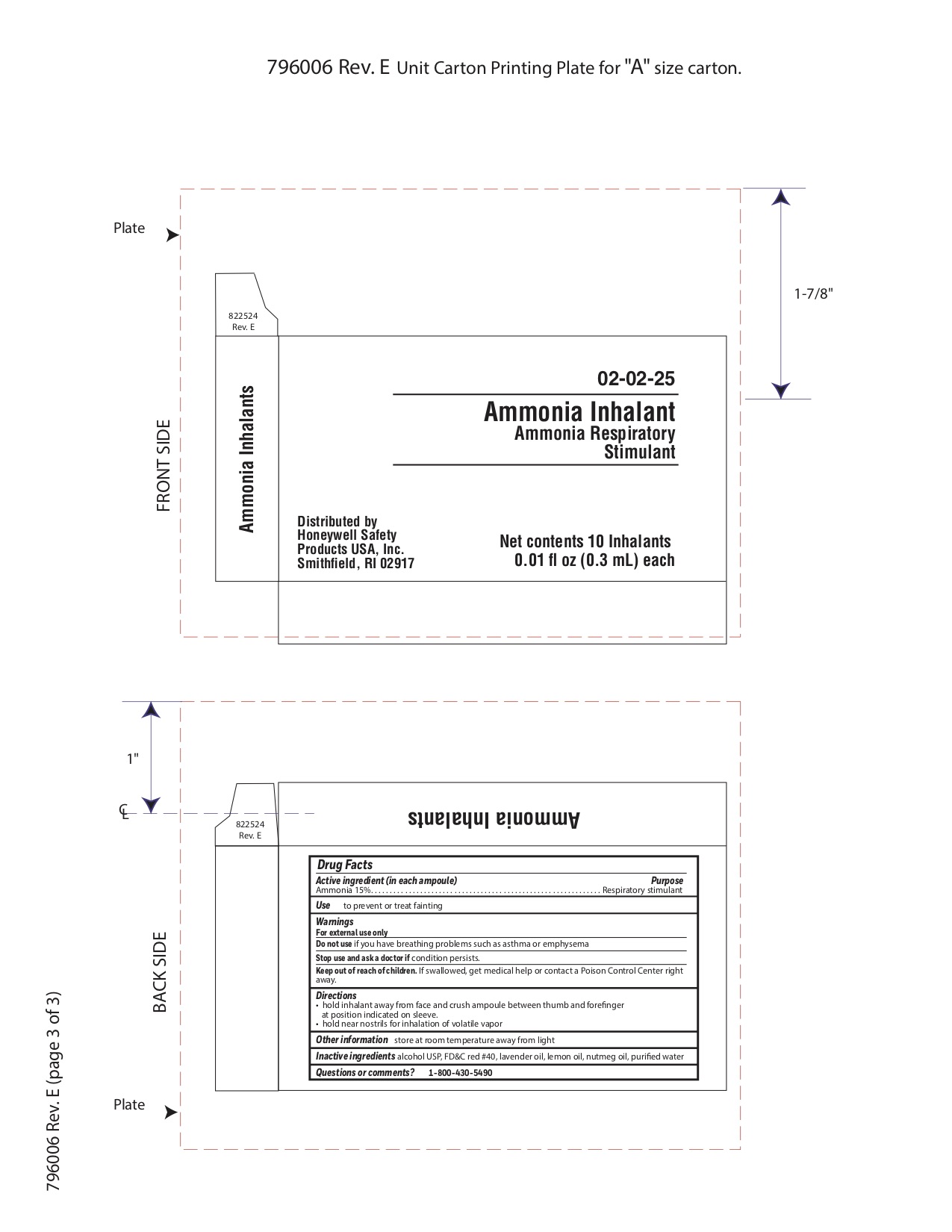
Ammonia
Active ingredient
Ammonia 15%
Ammonia
Purpose
Respiratory stimulant
Ammonia
Uses
- to prevent or treat fainting
Ammonia
Warnings
For external use only
Do not use
- if you have breathing problems such as asthma or emphysema
Stop use and ask a doctor if
Keep out of reach of children
- If swallowed get medical help or contact a Poison Control Center right away.
Ammonia
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia
Other information
- store at room temperature away from light
Ammonia
Inactive ingredient
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Ammonia
Questions or Comments?
1-800-430-5490
BZK
Active ingredient
Benzalkonium chloride 0.13% w/v
BZK
Purpose
First aid antiseptic
BZK
Uses
Antiseptic cleansing of face, hands, and body without soap and water
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
- if irritation, redness or other symptoms develop
- the condition persists or gets worse
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
BZK
Directions
tear open packet and use as a washcloth
BZK
Other information
- store at room temperature 15
0 to 30
0 C (5
0 - 86
0 F)
- do not reuse towelette
BZK
Inactiave ingredient
water
BzK
Questions
1-800-430-5490
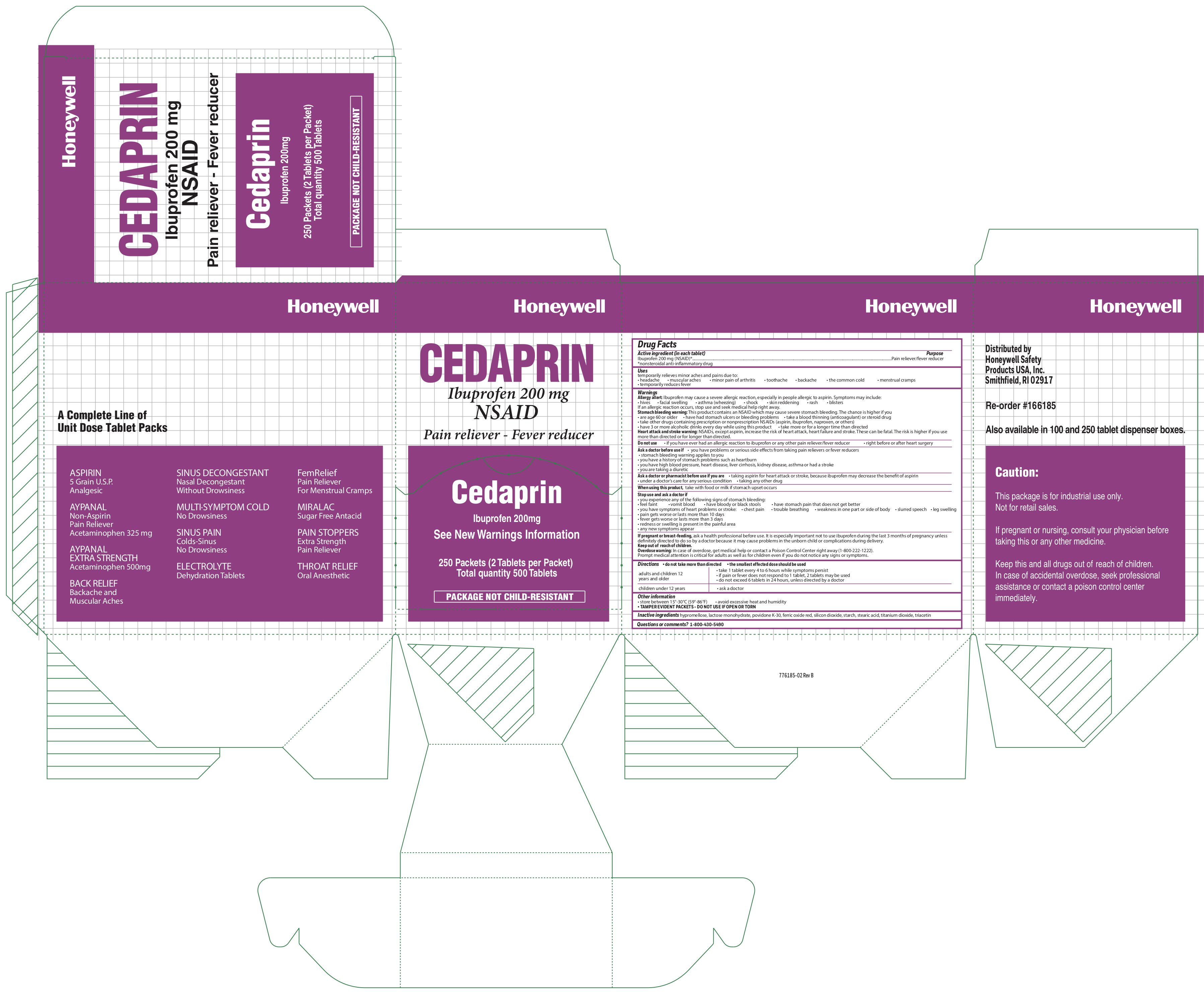
Cedaprin
Active ingredient
Ibuprofen 200 mg (NSAID)
*(nonsteroidal anti-inflammatory drug)
Cedaprin
Purpose
Pain reliever/fever reducer
Cedaprin
Uses
temporarily relieves minor aches and pains due to:
- headache
- muscular aches
- minor pain of arthritis
- toothache
- backache
- the common cold
- menstrual cramps
- temporarily reduces fever
Cedaprin
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Heart attack and stroke warning:
- NSAID's, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to ibuprofen or any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- you have problems or serious side effectsfrom taking pain relievers or fever reducers
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma or had a stroke
- you are taking a diuretic
Ask a doctor or a pharmacist before use if you are
- taking aspirin for heart attack or stroke, because ibuprofen may decrease the benefit of aspirin
- under a doctors care for any serious condition
- taking any other drug
When using this product,
take with food or milk if stomach upset occursv
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in oe part or side of body
- slurred speech
- leg swelling
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
If pregnant or breast-feeding
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children.
Overdose warning: In case of overdose, get medical help or contact a Poison Control Center right away(1-800-222-1222). Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Cedaprin
Directions
- do not take more than directed
- the smallest effective dose should be used
- adult and children 12 years of age and over:
- take 1 tablet every 4 to 6 hours while symptoms persist
- if pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours, unless directed by a doctor
- children under 12 years: ask a doctor
Cedaprin
Other information
- store between 15
0 -30
0 C (59
0 -86
0 F)
- avoid excessive heat and humidity
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
Cedaprin
Inactive ingredients
hypromellose, lactose monohydrate, opadry II 31K, povidone K-30, ferric oxide red, silicon dioxide, starch, stearic acid, titanium dioxide, triacetin
Questions or Comments?
1-800-430-590
Burn Jel
Active ingredient
Lidocaine HCl 2.0 %
Burn Jel
Purpoose
External analgesic
Burn Jel
Uses
temporarily relieves pain due to minor burns
Burn Jel
Warnings
For external use only
Do not use
- on large areas of the body, particularly over raw surfaces or blistered areas
Stop use and ask a doctor if
- the condition gets worse
- symptoms persist for more than 7 days
- condition clears up and recurs within a few dayseas
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Burn Jel
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Other information
- store at room temperature
- do not use if opened or torn
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water ...
Burn Jel
Questions
1-800-430-5490
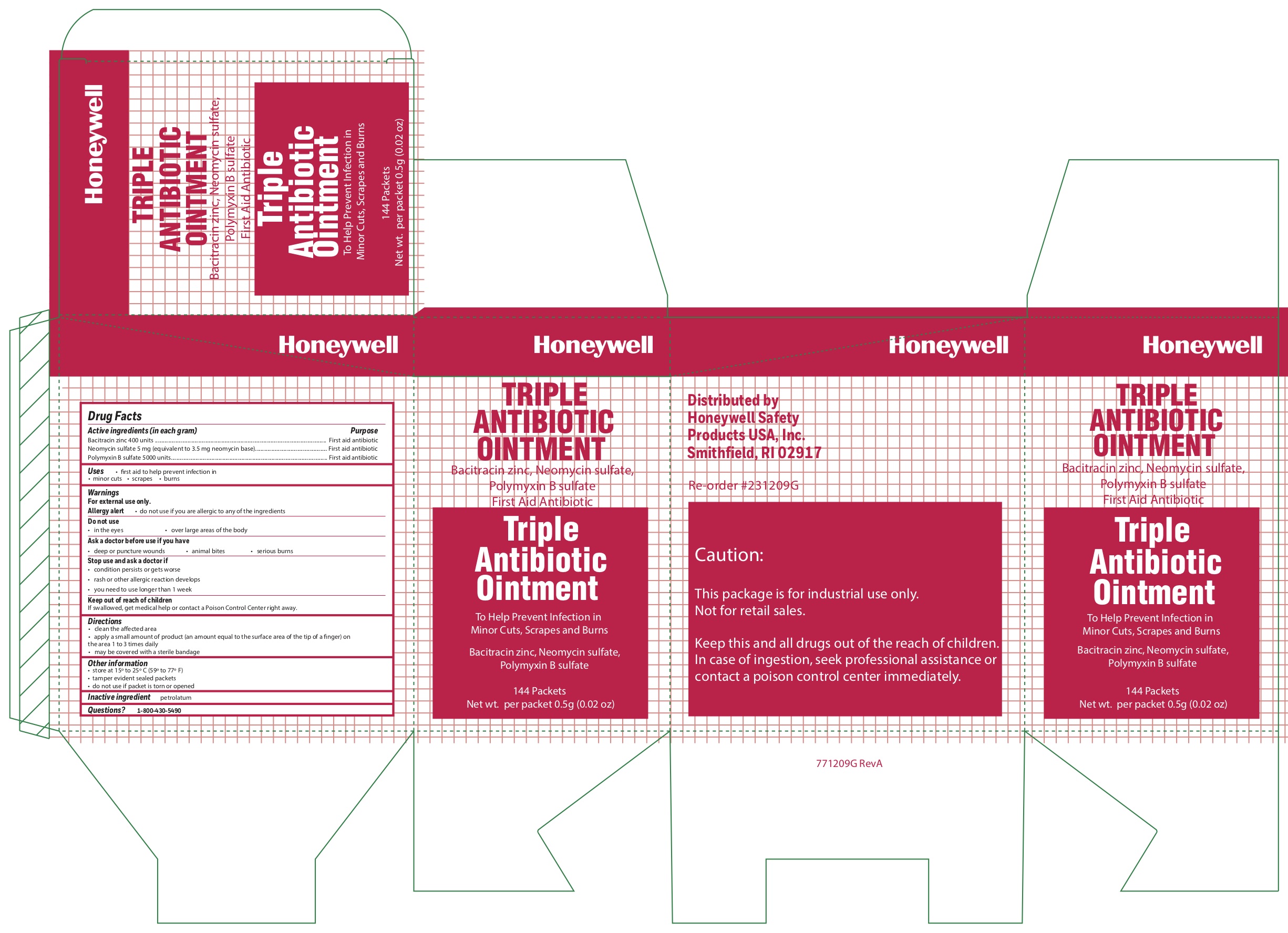
Triple
Active ingredients
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Purpose
First aid antibiotic
First aid antibiotic
First aid antibiotic
Triple
Uses
- first aid to help prevent infection in
- minor cuts
- scrapes
- burns
Triple
Warnings
For external use only
Allergy alert do not use if you are allergic to any of the ingredients
Do not use
in the eyes
over large areas of the body
Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burnsnot use if you are allergic to any of the ingredients
Stop use and ask a doctor if
- the condition persists or gets worse
- a rash or other allergic reaction develops
- you need to use longer than 1 week
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15
0 to 25
0 C (59
0 to 77
0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
Triple
Inactive ingredient
petrolatum
Triple
Questions?
1-800-430-5490
Aypanal
Active ingredient (in each tablet)
Acetaminophen 500 mg
Aypanal Ex
Purpose
Pain reliever/fever reducer
Aypanal Ex
Uses
- temporarily relieves minor aches and pains due to the common cold and headache
- temporarily reduces fever
Aypanal Ex
Warnings
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount.
- with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away
Do Not Use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
liver disease
Ask a doctor or pharmacist before use if
you are taking the blood thinning drug warfarin
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
If pregnant or breastfeeding,
ask a health professional before use.
Keep out of reach of children.
Overdose warning: In case ofl overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Aypanal Ex
Directions
- do not take more than directed (see overdose warning)
- adults and children 12 years of age and over: Take 2 tablets with water every 6 hours while symptoms last.
- do not take any more than 8 tablets in 24 hours.
- children under 12: consult a doctor
Aypanal Ex
Other information
- Store at room temperature 15
0-30
0 C (59
0 -86
0 F)
- TAMPER EVIDENT- DO NOT USE IF OPEN OR TORN
Aypanal Ex
Inactive ingredients
microcrystalline cellulose, povidone, sodium starch glycolate, starch, stearic acid
Aypanal Ex
Questions or Comments?
1-800-430-5490
4389
68P24AG KIT CONTENTS
1 TRIANGULAR BDG, NON-STERILE
3 INSTANT COLD PACK 4" X 6"
1 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 BURN JEL 1/8 OZ, 6 PER
1 NITRILE GLOVES 2PR BBP
1 ANTIMCRBL ANTSPTC TWLETTS
1 ELASTIC BANDAGE 3" X 4.5YD
1 CPR FILTERSHIELD 77-100
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 TRIPLE BIOTIC 12 PER ZIP BAG
1 CEDAPRIN 12 PACK PER ZIP BAG
1 aspirin12 PACK PER ZIP BAG
1 KIT PP 24 UNIT FA
1 ADHS TAPE .5"X2.5YD 2
1 GAUZE PADS 3"X3" 4/BX
3 AMMONIA INHALANT, BULK
Eyewash
Principal Display Panel

Aspirin
Principal Display Panel

Ammonia
Principal Display Panel
BZK
Principal Display Panel

Cedaprin
Principal Display Panel
Burn Jel
Principal Display Panel

Triple
Principal Display Panel
Aypanal EX
Principal Display Panel

4389 Kit Label
68P24AG









