LIVRELIEF PAIN RELIEF- capsaicin cream
LivCorp Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
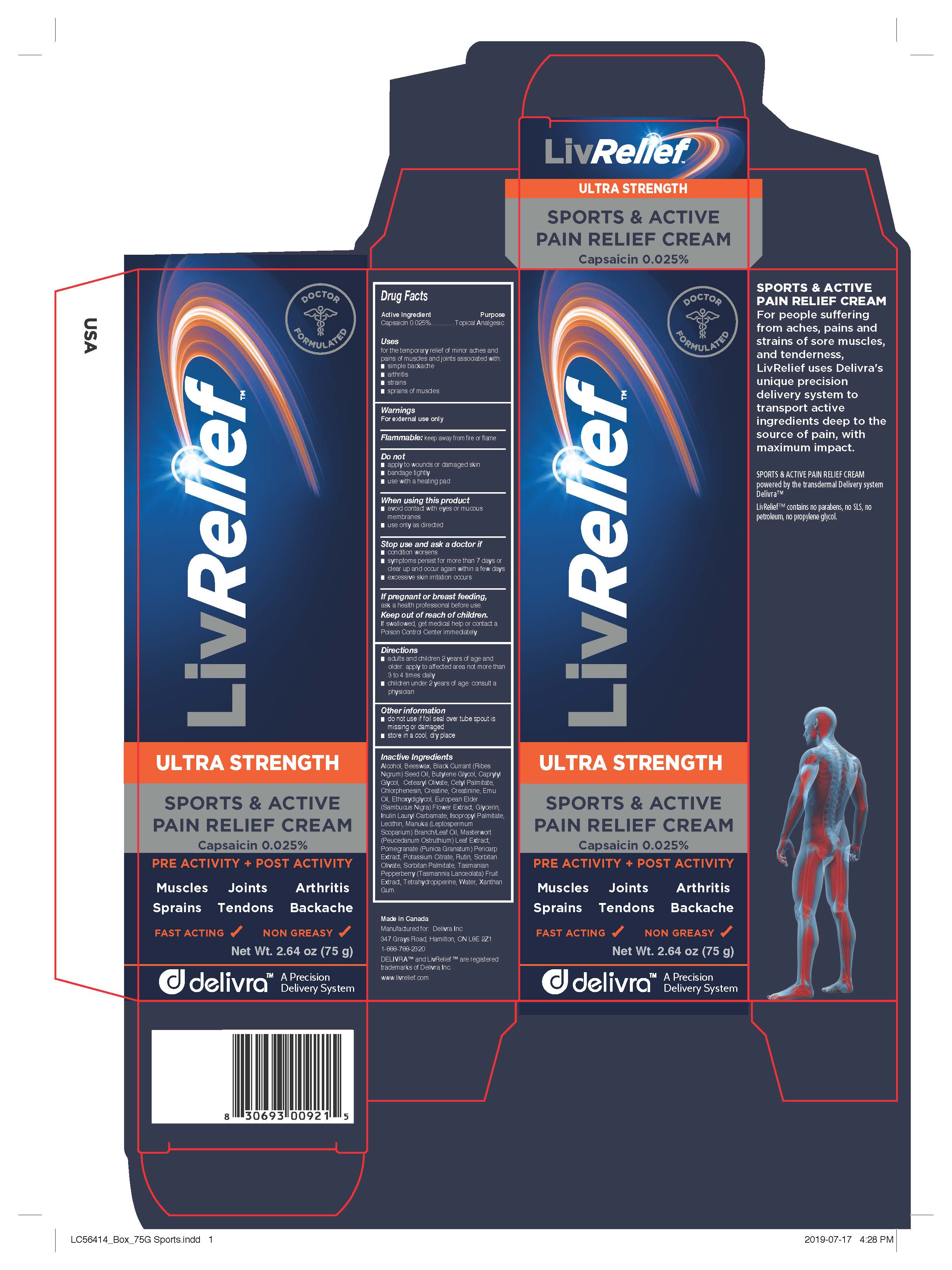
Drug Facts
Uses
for the temporary relief of minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- sprains
Warnings
For external use only.
Flammable: keep away from fire or flame.
Directions
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: consult a physician
Other information
- do not use if foil seal over tube spout is missing or damaged
- store in a cool, dry place
Inactive Ingredients
Alcohol, Beeswax, Black Currant (Ribes Nigrum) Seed Oil, Butylene Glycol, Caprylyl Glycol, Cetearyl Olivate, Cetyl Palmitate,
Chlorphenesin, Creatine, Creatinine, Emu Oil, Ethoxydiglycol, European Elder (Sambucus Nigra) Flower Extract, Glycerin,
Inulin Lauryl Carbamate, Isopropyl Palmitate, Lecithin, Manuka (Leptospermum Scoparium) Branch/Leaf Oil, Masterwort
(Peucedanum Ostruthium) Leaf Extract, Pomegranate (Punica Granatum) Pericarp Extract, Potassium Citrate, Rutin, Sorbitan
Olivate, Sorbitan Palmitate, Tasmanian Pepperberry (Tasmannia Lanceolata) Fruit Extract, Tetrahydropiperine, Water, Xanthan
Gum
Manufactured for LivCorp Inc.
Made in Canada
Manufactured for: Delivra Inc
347 Grays Road, Hamilton, ON L8E 2Z1
1-888-788-2320
DELIVRA™ and LivRelief™ are registered
trademarks of Delivra Inc.
www.livrelief.com
Side Panel
SPORTS & ACTIVE
PAIN RELIEF CREAM
For people suffering from aches, pains and strains of sore muscles, and tenderness, LivRelief uses Delivra's unique precision delivery system to transport active ingredients deep to the source of pain, with maximum impact.\
SPORTS & ACTIVE PAIN RELIEF CREAM
powered by the transdermal Delivery system
Delivra™
LivRelief™ contains no parabens, no SLS, no
petroleum, no propylene glycol.
| LIVRELIEF
PAIN RELIEF
capsaicin cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - LivCorp Inc. (248554748) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Les Emballages Facoteck Inc | 245423439 | manufacture(42903-010) | |