Label: CEFOTAXIME- cefotaxime injection powder, for solution
- NDC Code(s): 21586-011-01, 21586-011-02, 21586-012-01, 21586-012-02
- Packager: SteriMax Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 13, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
IMPORTANT PRESCRIBING INFORMATION
January 13, 2023
Temporary Importation of Cefotaxime for Injection to Address Drug Shortage
Dear Healthcare Professional:
Due to the current critical shortage of Cefotaxime for Injection products in the United States (U.S.) market, SteriMax Inc. (SteriMax), in conjunction with Provepharm, Inc. (Provepharm) and Direct Success, Inc. (Direct Success) is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of the drug. SteriMax has initiated temporary importation of non-FDA approved Cefotaxime for Injection (1 g/vial, and 2 g/vial) into the U.S. market. The Cefotaxime for Injection from SteriMax is marketed in Canada and is manufactured at an FDA-inspected facility that complies with current Good Manufacturing Practice requirements.
At this time, no other entity except Provepharm or its distributor Direct Success is authorized by the FDA to import or distribute SteriMax’s Cefotaxime for Injection in the United States. FDA has not approved SteriMax’s Cefotaxime for Injection in the United States.
Effective immediately, Provepharm will distribute the following presentations of SteriMax’s Cefotaxime for Injection to address the critical shortage:
Note: DIN refers to Drug Identification Number for products approved by Health Canada SteriMax Cefotaxime for Injection 1 g/vial (as cefotaxime sodium) DIN: 02434091
(Canada)NDC 21586-011-2 2 g/vial (as cefotaxime sodium) DIN: 02434105
(Canada)NDC 21586-012-2 The barcode on the imported product label may not register accurately on the U.S. scanning systems. Institutions should manually input the imported product information into their systems and confirm that the barcode, if scanned, provides correct information. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
In addition, the packaging of the imported product does not include serialization information. SteriMax’s Cefotaxime for Injection does not meet the Drug Supply Chain Security Act (DSCSA) requirements for the Interoperable Exchange of Information for Tracing of Human, Finished Prescription Drugs.
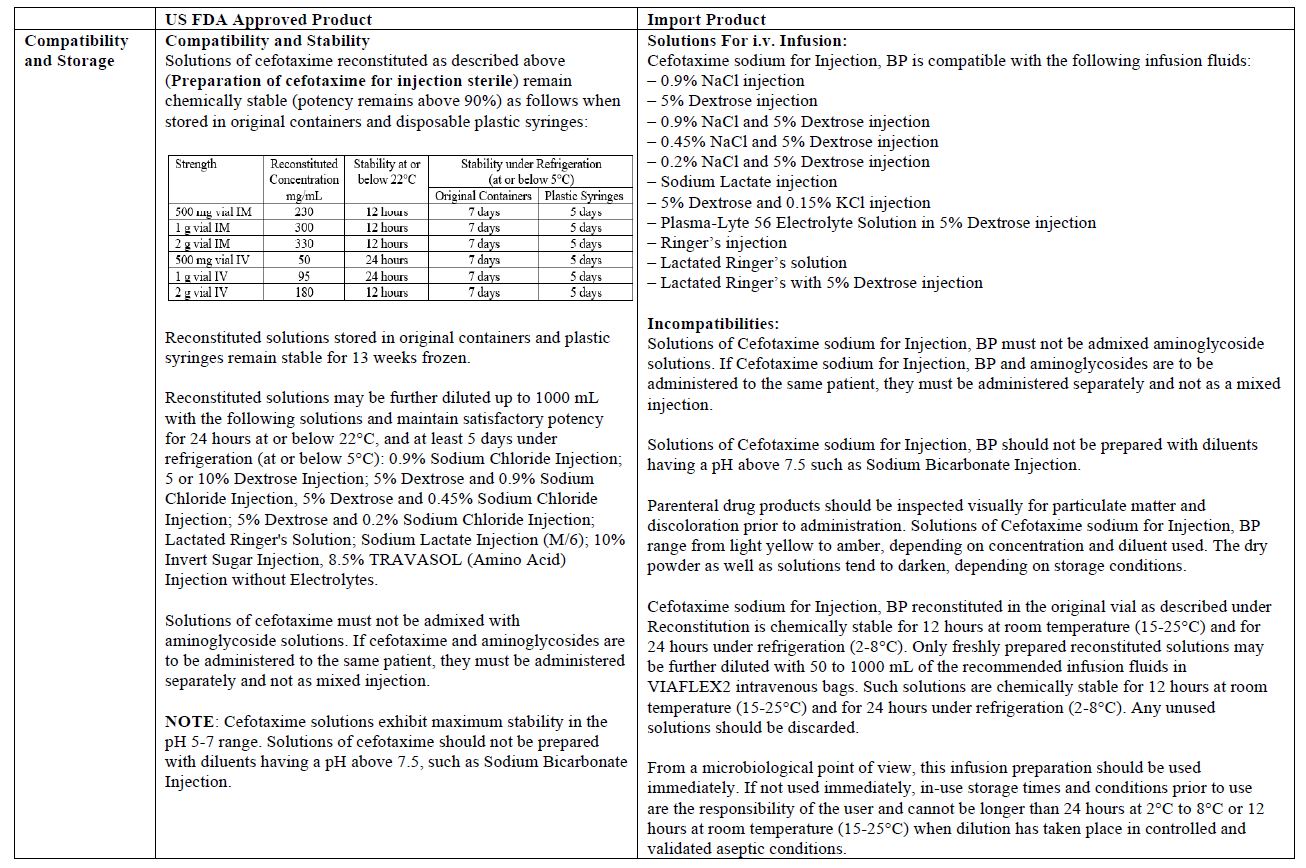
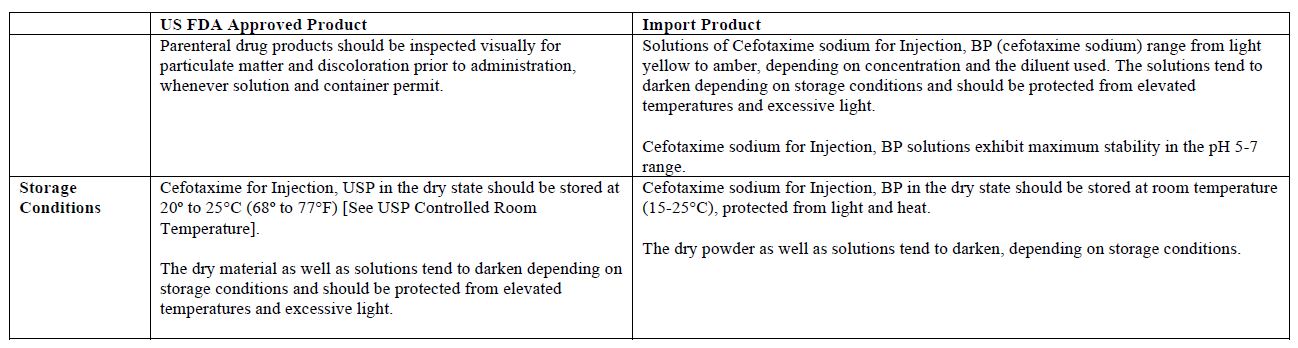
The vial and carton labels will display the text used and approved for marketing the products in Canada with both English and French translations. It is important to note that there are differences in the format and content of the labeling between the US approved product and SteriMax’s Cefotaxime for Injection. Please see the product comparison tables at the end of this letter.
Cefotaxime for Injection is available only by prescription in the U.S. Please refer to the package insert for the FDA-approved Cefotaxime for Injection drug product for full prescribing information.
Finally, please ensure that your staff and others in your institution who may be involved in the administration of Cefotaxime for Injection receive a copy of this letter and review the information.
If you have any questions about the information contained in this letter, any quality related problems, or questions on the use of SteriMax’s Cefotaxime for Injection, please contact SteriMax Inc. Customer Service at 1-800-881-3550.
To place an order, please contact Direct Success at Distribution@DSuccess.com or 1-877-404-3338.
Healthcare providers should report adverse events associated with the use of SteriMax’s Cefotaxime for Injection to Provepharm at 1-833-684-3234.
Adverse events or quality problems experienced with the use of this product may also be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, by regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
We remain at your disposal to answer any questions you may have about our product; and provide more information if needed.
Sincerely,

Ritesh Acharya
Executive Vice President, Scientific Affairs
SteriMax Inc.
- Cefotaxime for Injection - 1 g per vial
- Cefotaxime for Injection - 2 g per vial
-
INGREDIENTS AND APPEARANCE
CEFOTAXIME
cefotaxime injection powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21586-011 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFOTAXIME SODIUM (UNII: 258J72S7TZ) (CEFOTAXIME - UNII:N2GI8B1GK7) CEFOTAXIME 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21586-011-02 10 in 1 PACKAGE 08/01/2019 1 NDC:21586-011-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 08/01/2019 CEFOTAXIME
cefotaxime injection powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21586-012 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFOTAXIME SODIUM (UNII: 258J72S7TZ) (CEFOTAXIME - UNII:N2GI8B1GK7) CEFOTAXIME 2 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21586-012-02 10 in 1 PACKAGE 08/01/2019 1 NDC:21586-012-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 08/01/2019 Labeler - SteriMax Inc. (251574851)




