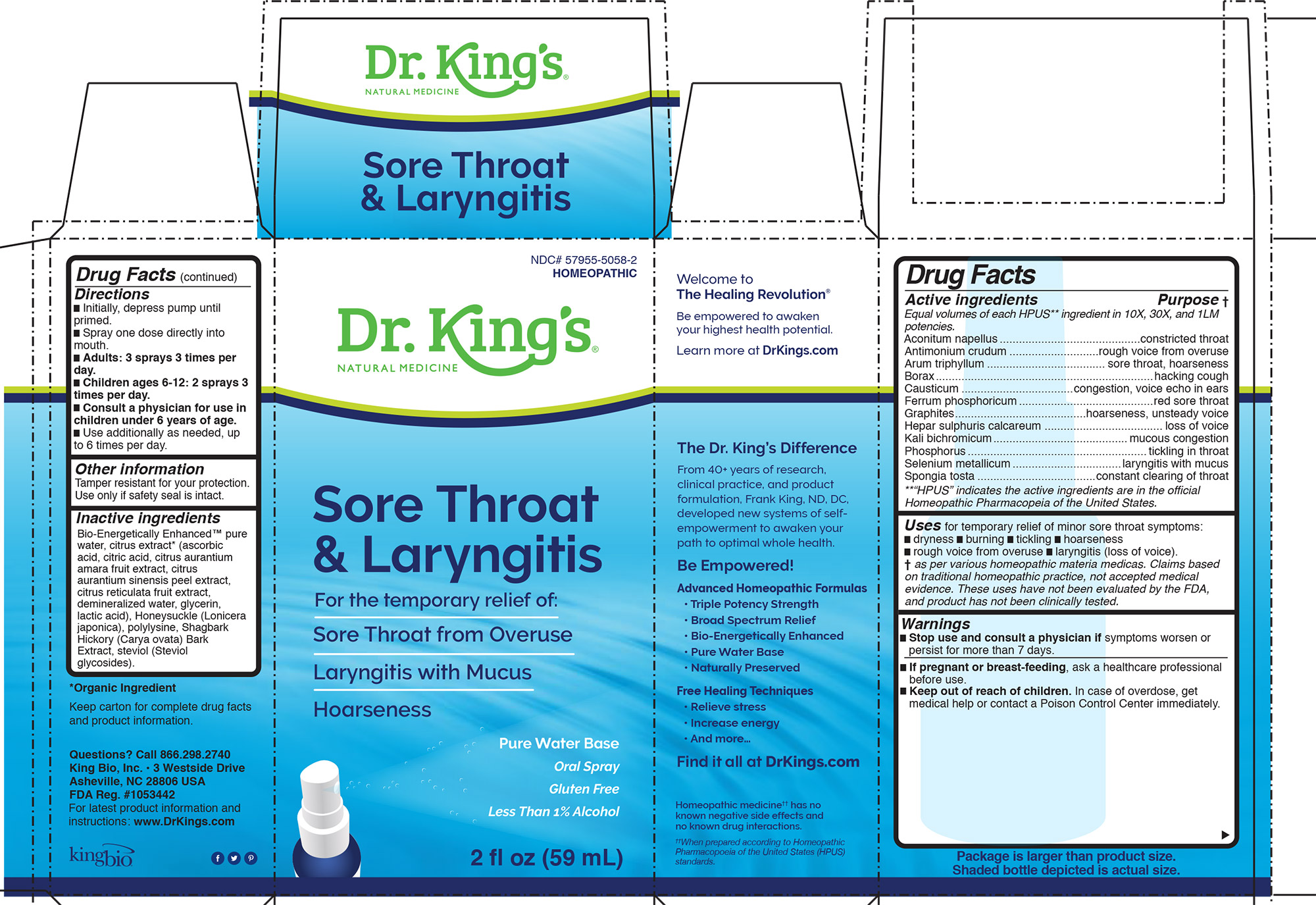
Label: SORE THROAT AND LARYNGITIS- aconitum napellus, antimonium crudum, arum triphyllum, borax, causticum, ferrum phosphoricum, graphites, hepar sulphuris calcareum, kali bichromicum, phosphorus, selenium metallicum, spongia tosta liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-5058-2 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 9, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INDICATIONS & USAGE
Uses for temporary relief of minor sore throat symptoms:
- dryness
- burning
- tickling
- hoarseness
- rough voice from overuse
- laryngitis (loss of voice).
† as per various homeopathic materia medicas. Claims based
on traditional homeopathic practice, not accepted medical
evidence. These uses have not been evaluated by the FDA,
and product has not been clinically tested. - WARNINGS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive Ingredients:
Bio-Energetically Enhanced™ pure
water, citrus extract* (ascorbic
acid, citric acid, citrus aurantium
amara fruit extract, citrus
aurantium sinensis peel extract,
citrus reticulata fruit extract,
demineralized water, glycerin,
lactic acid), Honeysuckle (Lonicera
japonica), polylysine, Shagbark
Hickory (Carya ovata) Bark
Extract, steviol (Steviol
glycosides). -
PURPOSE
HPUS active ingredients Purpose
Equal volumes of each HPUS** ingredient in 10X, 30X, and 1LM
potencies.
Aconitum napellus............................................constricted throat
Antimonium crudum ............................rough voice from overuse
Arum triphyllum ..................................... sore throat, hoarseness
Borax....................................................................hacking cough
Causticum ...................................congestion, voice echo in ears
Ferrum phosphoricum..........................................red sore throat
Graphites.........................................hoarseness, unsteady voice
Hepar sulphuris calcareum ..................................... loss of voice
Kali bichromicum..........................................mucous congestion
Phosphorus........................................................tickling in throat
Selenium metallicum..................................laryngitis with mucus
Spongia tosta .....................................constant clearing of throat
**“HPUS” indicates the active ingredients are in the official
Homeopathic Pharmacopeia of the United States. - QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SORE THROAT AND LARYNGITIS
aconitum napellus, antimonium crudum, arum triphyllum, borax, causticum, ferrum phosphoricum, graphites, hepar sulphuris calcareum, kali bichromicum, phosphorus, selenium metallicum, spongia tosta liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-5058 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 10 [hp_X] in 59 mL ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 10 [hp_X] in 59 mL ARISAEMA TRIPHYLLUM ROOT (UNII: DM64K844DM) (ARISAEMA TRIPHYLLUM ROOT - UNII:DM64K844DM) ARISAEMA TRIPHYLLUM ROOT 10 [hp_X] in 59 mL SODIUM BORATE (UNII: 91MBZ8H3QO) (BORATE ION - UNII:44OAE30D22) SODIUM BORATE 10 [hp_X] in 59 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 10 [hp_X] in 59 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 10 [hp_X] in 59 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 10 [hp_X] in 59 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 10 [hp_X] in 59 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 10 [hp_X] in 59 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 10 [hp_X] in 59 mL SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 10 [hp_X] in 59 mL SPONGIA OFFICINALIS SKELETON, ROASTED (UNII: 1PIP394IID) (SPONGIA OFFICINALIS SKELETON, ROASTED - UNII:1PIP394IID) SPONGIA OFFICINALIS SKELETON, ROASTED 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARYA OVATA BARK (UNII: X765CF609L) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) POLY-L-LYSINE (30000-70000 MW) (UNII: 0A1V8JTU2M) REBAUDIOSIDE A (UNII: B3FUD0528F) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-5058-2 1 in 1 CARTON 02/22/2018 1 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/22/2018 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture(57955-5058) , api manufacture(57955-5058)