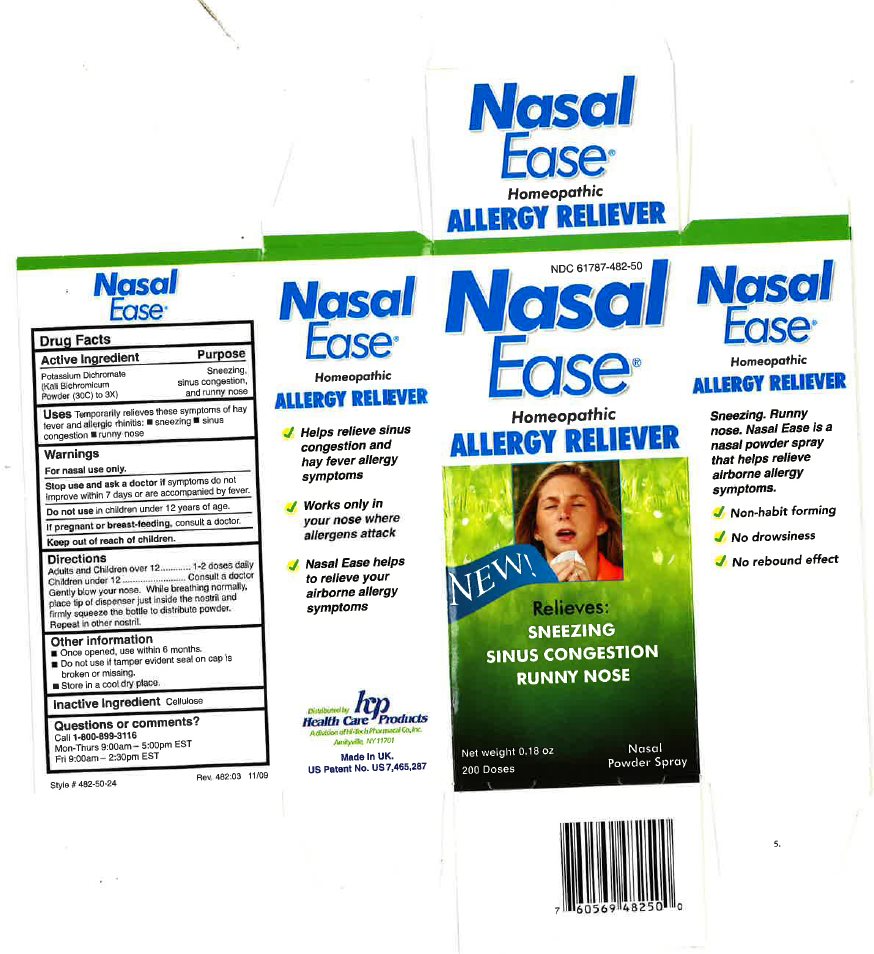
NASAL EASE ALLERGY RELIEVER- potassium dichromate powder
Health Care Products
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts
Uses
Temporary relieves three symptoms of hay fever and allergic rhinitis;
Sneezing;
Sinus congestion;
Runny nose;
Directions
Adults and Children over 12 ........ 1-2 doses daily
Children under 12 ........ Consult a doctor
Gently blow your nose. While breathing normally, place tip of dispenser just inside the nostril and firmly squeeze the bottle to distribute powder. Repeat in other nostril. Once opened, use within 6 months. Do not use if tamper evident seal on bottle is broken or missing. Store in a cool dry place.
| NASAL EASE ALLERGY RELIEVER
potassium dichromate powder |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Health Care Products (101196749) |
| Registrant - Hi-Tech Pharmacal Co., Inc. (101196749) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nasaleze LTD | 233326748 | MANUFACTURE(61787-482) | |