Label: BETHANECHOL CHLORIDE tablet
-
Contains inactivated NDC Code(s)
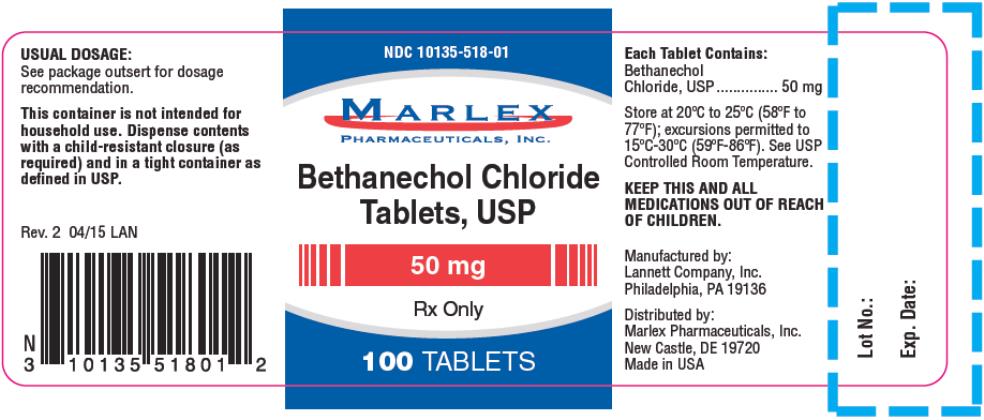
NDC Code(s): 10135-516-01, 10135-517-01, 10135-518-01 - Packager: Marlex Pharmaceuticals Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 28, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Bethanechol chloride, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related to acetylcholine.
It is designated chemically as 2-[(aminocarbonyl)oxy]-N, N, N,-trimethyl-1-propanaminium chloride. Its molecular formula is C7H17CIN2O2 and its structural formula is:
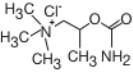
It is a white, hygroscopic crystalline powder having a slight amine-like odor, freely soluble in water, and has a molecular weight of 196.68.
Each tablet for oral administration contains 5 mg, 10 mg, 25 mg or 50 mg bethanechol chloride, USP. Tablets also contain the following inactive ingredients: microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, talc, and magnesium stearate; the 50 mg tablet also contains D&C Yellow #10 aluminum lake.
-
CLINICAL PHARMACOLOGY:
Bethanechol chloride acts principally be producing the effects of stimulation of the parasympathetic nervous system. It increases the tone of the detrusor urinae muscle, usually producing a contraction sufficiently strong to initiate micturition and empty the bladder. It stimulates gastric motility, increases gastric tone and often restores impaired rhythmic peristalsis.
Stimulation of the parasympathetic nervous system releases acetylcholine at the nerve endings. When spontaneous stimulation is reduced and therapeutic intervention is required, acetylcholine can be given, but it is rapidly hydrolyzed by cholinesterase and its effects are transient. Bethanechol chloride is not destroyed by cholinesterase and its effects are more prolonged than those of acetylcholine.
Effects on the GI and urinary tracts sometimes appear within 30 minutes after oral administration of bethanechol chloride, but more often 60 to 90 minutes are required to reach maximum effectiveness. Following oral administration, the usual duration of action of bethanechol is one hour, although large doses (300 to 400 mg) have been reported to produce effects for up to six hours. Subcutaneous injection produces a more intense action on bladder muscle than does administration of the drug.
Because of the selective action of bethanechol, nicotinic symptoms of chlolinergic stimulation are usually absent or minimal when orally or subcutaneously administered in therapeutic doses, while muscarinic effects are prominent. Muscarinic effects usually occur within 5 to 15 minutes after subcutaneous injection, reach a maximum in 15 to 30 minutes, and disappear within two hours. Doses that stimulate micturition and defecation and increase peristalsis do not ordinarily stimulate ganglia or voluntary muscles. Therapeutic test doses in normal human subjects have little effect on heart rate, blood pressure or peripheral circulation.
Bethanechol chloride does not cross the blood-brain barrier because of its charged quaternary amine moiety. The metabolic rate and mode of excretion of the drug have not been elucidated.
A clinical study (Diokno, A.C.; Lapides, J.; Urol 10: 23-24, July 1977) was conducted on the relative effectiveness of oral and subcutaneous doses of bethanechol chloride on the stretch response of bladder muscle in patients with urinary retention. Results showed that 5 mg of the drug given subcutaneously stimulated a response that was more rapid in onset and of larger magnitude than an oral dose of 50 mg, 100 mg, or 200 mg. All the oral doses, however, had a longer duration of effect than the subcutaneous dose. Although the 50 mg oral dose caused little change in intravesical pressure in this study, this dose has been found in other studies to be clinically effective in the rehabilitation of patients with decompensated bladders.
- INDICATIONS AND USAGE:
-
CONTRAINDICATIONS:
Hypersensitivity to bethanechol chloride tablets, hyperthyroidism, peptic ulcer, latent or active bronchial asthma, pronounced bradycardia or hypotension, vasomotor instability, coronary artery disease, epilepsy and parkinsonism.
Bethanechol chloride should not be employed when the strength or integrity of the gastrointestinal or bladder wall is in question, or in the presence of mechanical obstruction; when increased muscular activity of the gastrointestinal tract or urinary bladder might prove harmful, as following recent urinary bladder surgery, gastrointestinal resection and anastomosis, or when there is possible gastrointestinal obstruction; in bladder neck obstruction, spastic gastrointestinal disturbances, acute inflammatory lesions of the gastrointestinal tract, or peritonitis; or in marked vagotonia.
-
PRECAUTIONS:
General:
In urinary retention, if the sphincter fails to relax as bethanechol contracts the bladder, urine may be forced up the ureter into the kidney pelvis. If there is bacteriuria, this may cause reflux infection.
Information for Patients:
Bethanechol chloride tablets should preferably be taken one hour before or two hours after meals to avoid nausea or vomiting. Dizziness, lightheadedness or fainting may occur, especially when getting up from a lying or sitting position.
Drug Interactions:
Special care is required if this drug is given to patients receiving ganglion blocking compounds because a critical fall in blood pressure may occur. Usually, severe abdominal symptoms appear there in such a fall in the blood pressure.
Carcinogeneis, Mutagenesis, Impairment of Fertility:
Long-term studies in animals have not been performed to evaluate the effects upon fertility, mutagenic or carcinogenic potential of bethanechol chloride.
Pregnancy: Teratogenic Effects:
Pregnancy Category C. Animal reproduction studies have not been conducted with bethanechol chloride. It is also not known whether bethanechol chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Bethanechol chloride should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
It is not known whether this drug is secreted in human milk. Because many drugs are secreted in human milk and because of the potential for serious adverse reactions from bethanechol chloride in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS:
Adverse reactions are rare following oral administration of bethanechol, but are more common following subcutaneous injection. Adverse reactions are more likely to occur when dosage is increased.
The following adverse reactions have been observed: Body as a Whole: malaise; Digestive: abdominal cramps or discomfort, colicky pain, nausea and belching, diarrhea, borborygmi, salivation; Renal: urinary urgency; Nervous System: headache; Cardiovascular: a fall in blood pressure with reflux tachycardia, vasomotor response; Skin: flushing producing a feeling of warmth, sensation of heat about the face, sweating; Respiratory: bronchial constriction, asthmatic attacks; Special Senses: lacrimation, miosis.
Causal Relationship Unknown: The following adverse reactions have been reported, and a causal relationship to therapy with bethanechol has not been established: Body as a whole: malaise; Nervous System: seizures.
-
OVERDOSAGE:
Early signs of overdosage are abdominal discomfort, salivation, flushing of the skin (“hot feeling”), sweating, nausea, and vomiting.
Atropine Sulfate is a specific antidote. The recommended dose for adults is 0.6 mg. Repeat doses can be given every two hours, according to clinical response. The recommended dosage in infants and children up to 12 years of age is 0.01 mg/kg (to a maximum single dose of 0.4 mg) repeated every two hours as needed until the desired effect is obtained or adverse effects of atropine preclude further usage. Subcutaneous injection of atropine is preferred except in emergencies when the intravenous route may be employed.
The oral LD50 of bethanechol chloride is 1510 mg/kg in the mouse.
-
DOSAGE AND ADMINISTRATION:
Dosage must be individualized, depending on the type and severity of the condition to be treated.
Preferably give the drug when the stomach is empty. If taken soon after eating, nausea and vomiting may occur.
The usual adult oral dose ranges from 10 to 50 mg three or four times a day. The minimum effective dose is determined by giving 5 to 10 mg initially and repeating the same amount at hourly intervals until satisfactory response occurs, or until a maximum of 50 mg has been given. The effects of the drug sometimes appear within 30 minutes and are usually maximal within 60 to 90 minutes. The drug effects persist for about one hour.
If necessary, the effects of the drug can be abolished promptly by atropine (see OVERDOSAGE).
-
HOW SUPPLIED:
Bethanechol Chloride Tablets are supplied as follows:
5 mg tablets: off-white, round, flat-faced beveled edge, bisected tablets debossed LCI over 1332 on one side and plain on the other side.
They are supplied in bottles of 100 tablets; NDC 10135-0515-01.
10 mg tablets: off-white, round, flat-faced beveled edge, bisected tablets debossed LCI over 1340 on one side and plain on the other side.
They are supplied in bottles of 100 tablets; NDC 10135-0516-01.
25 mg tablets: off-white, round, flat-faced beveled edge, bisected tablets debossed LCI over 1356 on one side and plain on the other side.
They are supplied in bottles of 100 tablets; NDC 10135-0517-01.
50 mg tablets: off-white, round, flat-faced beveled edge, bisected tablets debossed LCI over 1329 on one side and plain on the other side.
They are supplied in bottles of 100 tablets; NDC 10135-0518-01.
This container not intended for household use. Dispense contents with a child-resistant closure (as required) and in a tight container as defined in the USP.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature].
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
Distributed by:
Marlex Pharmaceuticals, Inc.
New Castle, DE 19720
Manufactured by:
Lannett Company, Inc.
Philadelphia, PA 19136
RX Only
Made in the USA
Rev. 2 4/15 LAN
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BETHANECHOL CHLORIDE
bethanechol chloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10135-516 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETHANECHOL CHLORIDE (UNII: H4QBZ2LO84) (BETHANECHOL - UNII:004F72P8F4) BETHANECHOL CHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE B POTATO (UNII: 27NA468985) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (off-white) Score 2 pieces Shape ROUND (flat-faced beveled edge) Size 10mm Flavor Imprint Code LCI;1340 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10135-516-01 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040704 04/01/2015 BETHANECHOL CHLORIDE
bethanechol chloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10135-517 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETHANECHOL CHLORIDE (UNII: H4QBZ2LO84) (BETHANECHOL - UNII:004F72P8F4) BETHANECHOL CHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE B POTATO (UNII: 27NA468985) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (off-white) Score 2 pieces Shape ROUND (flat-faced beveled edge) Size 10mm Flavor Imprint Code LCI;1356 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10135-517-01 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040678 04/01/2015 BETHANECHOL CHLORIDE
bethanechol chloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10135-518 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETHANECHOL CHLORIDE (UNII: H4QBZ2LO84) (BETHANECHOL - UNII:004F72P8F4) BETHANECHOL CHLORIDE 50 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE B POTATO (UNII: 27NA468985) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color YELLOW Score 2 pieces Shape ROUND (flat-faced beveled edge) Size 11mm Flavor Imprint Code LCI;1329 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10135-518-01 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040677 04/01/2015 Labeler - Marlex Pharmaceuticals Inc (782540215)