LAMISIL AT- terbinafine hydrochloride cream
REMEDYREPACK INC.
----------
Lamisil AT ®
Uses
- cures most athlete's foot (tinea pedis)
- cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- relieves itching, burning, cracking and scaling which accompany these conditions
Directions
| 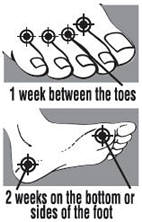 |
Other information
- do not use if seal on tube is broken or is not visible
- store at controlled room temperature 20°-25°C (68°-77°F)
Inactive ingredients
benzyl alcohol, cetyl alcohol, cetyl palmitate, isopropyl myristate, polysorbate 60, purified water, sodium hydroxide, sorbitan monostearate, stearyl alcohol
DRUG: Lamisil AT
GENERIC: Terbinafine Hydrochloride
DOSAGE: CREAM
ADMINSTRATION: TOPICAL
NDC: 70518-1736-0
COLOR: white
PACKAGING: 30 g in 1 TUBE
OUTER PACKAGING: 1 in 1 CARTON
ACTIVE INGREDIENT(S):
- Terbinafine Hydrochloride 1g in 100g
INACTIVE INGREDIENT(S):
- benzyl alcohol
- sodium hydroxide
- sorbitan monostearate
- water
- polysorbate 60
- cetyl alcohol
- cetyl palmitate
- isopropyl myristate
- stearyl alcohol

| LAMISIL AT
terbinafine hydrochloride cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |
Revised: 5/2020
Document Id: a6cb0816-1559-c572-e053-2a95a90a8feb
Set id: 8d2a3b91-2872-40d0-a962-ebf90cf17986
Version: 4
Effective Time: 20200529
REMEDYREPACK INC.