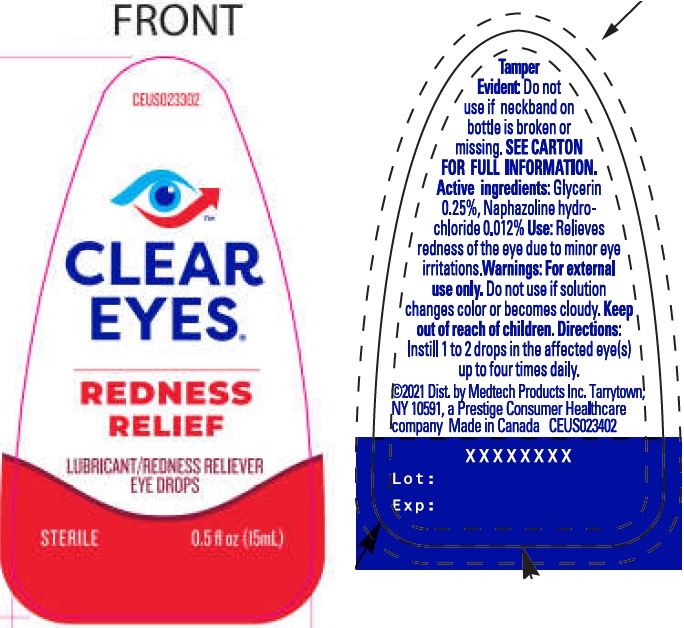
CLEAR EYES REDNESS RELIEF EYE DROPS STERILE- glycerin, naphazoline hydrochloride liquid
Geodis Logistics LLC
----------
Clear Eyes Redness Relief Eye Drops Sterile
Uses
- for the relief of redness of the eye due to minor eye irritations
- for the temporary relief of burning and irritations due to dryness of the eye
- for use as a protectant against further irritation or to relieve dryness of the eye
Warnings
For external use only.
When using this product
- to avoid contamination, do not touch tip to any surface
- replace cap after using
- overuse may produce increased redness of the eye
- pupils may become temporarily enlarged
| CLEAR EYES REDNESS RELIEF EYE DROPS STERILE
glycerin, naphazoline hydrochloride liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Geodis Logistics LLC (877844097) |
Revised: 11/2023
Document Id: 09d6b845-ce49-6bac-e063-6394a90a1369
Set id: 8c98dd44-a689-4f3a-b6ff-51401d0849ce
Version: 2
Effective Time: 20231110
Geodis Logistics LLC